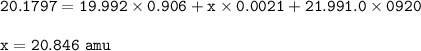Chemistry, 15.02.2022 03:40 jellyangie1
neon has 3 different isotopes, neon-20, neon-21, and neon-22. the first isotope has a mass 19.992 amu and an abundance of 90.60% . the second isotope (neon-21) has an abundance of 0.21%. the third isotope has a mass 21.991 amu and an abundance of 9.20%. what is the mass of the second isotope?

Answers: 2


Another question on Chemistry



Chemistry, 22.06.2019 16:00
Which of the following is the correct definition of chemical energy? a. energy an object has because of its motion or position b. energy resulting from the flow of charged particles, such as electrons or ions c. energy produced from the splitting of atoms d. energy stored in chemical bonds of molecules
Answers: 1

Chemistry, 22.06.2019 21:30
The solid xy decomposes into gaseous x and y: xy(s) m x(g) + y(g) kp = 4.1 (at 0 °c) if the reaction is carried out in a 22.4 l container, which initial amounts of x and y will result in the formation of solid xy?
Answers: 1
You know the right answer?
neon has 3 different isotopes, neon-20, neon-21, and neon-22. the first isotope has a mass 19.992 am...
Questions



Mathematics, 26.07.2020 09:01

Mathematics, 26.07.2020 09:01

Mathematics, 26.07.2020 09:01

Mathematics, 26.07.2020 09:01

Mathematics, 26.07.2020 09:01

Mathematics, 26.07.2020 09:01

Mathematics, 26.07.2020 09:01

Mathematics, 26.07.2020 14:01