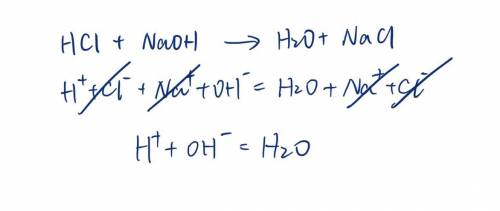Chemistry, 13.02.2022 23:50 ralphmillerrr
. A strong acid, HCl, is titrated with a strong base, NaOH. Write the net ionic equation for the reaction. Do not include spectator ions in the equation.

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 09:30
In apex! a liquid heated beyond a certain temperature becomes
Answers: 1

Chemistry, 22.06.2019 16:50
Which of the following is an indication that a substance has undergone a chemical change? a. no new product has been formed. b. the color of the substance has not changed. c. the original constitute has not changed. d. the molecular structure has changed.
Answers: 1

Chemistry, 22.06.2019 17:30
I'm learning about the periodic tables and what each subject's configuration is. for example, hydrogen is 1s^1, but i don't understand how you get that. can someone me understand how to figure out how to figure this out? sorry if the question makes no sense, but it would really a lot if you could me understand! you so much if you can!
Answers: 1
You know the right answer?
. A strong acid, HCl, is titrated with a strong base, NaOH. Write the net ionic equation for the rea...
Questions


Mathematics, 12.10.2020 21:01

History, 12.10.2020 21:01



Mathematics, 12.10.2020 21:01

Mathematics, 12.10.2020 21:01

English, 12.10.2020 21:01

Biology, 12.10.2020 21:01


Chemistry, 12.10.2020 21:01

History, 12.10.2020 21:01

Physics, 12.10.2020 21:01

Mathematics, 12.10.2020 21:01

Mathematics, 12.10.2020 21:01

Mathematics, 12.10.2020 21:01




Mathematics, 12.10.2020 21:01