Chemistry, 13.02.2022 09:20 victoria8281
Determine the molar mass of (show your work) :
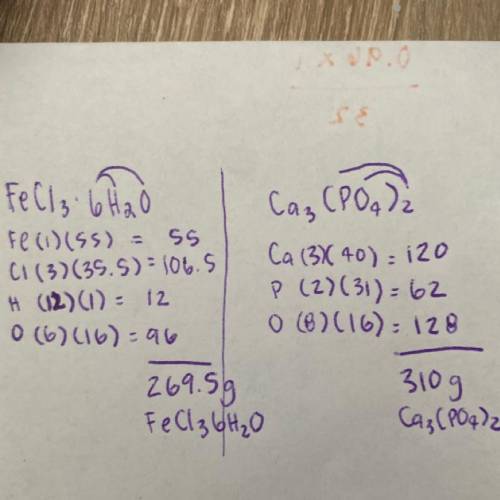
FeCl3•6H2O (note, this is all 1 molecule. Its an FeCl3 with a cage of 6H2O’s attached. These molecules are known as hydrates.)
Ca3(PO4)2

Answers: 2


Another question on Chemistry


Chemistry, 23.06.2019 02:30
When the ionic compound nabr dissolves in water, br– ions are pulled into solution by the attraction between what two particles? a. the na+ and br– ions b. the na+ ion and the negative end of a water molecule c. the br– ion and the positive end of a water molecule d. the br– ion and the negative end of a water molecule
Answers: 1


Chemistry, 23.06.2019 04:10
An unknown substance has been shown to have metallic bonds. which of the following is most likely a property of this substance? a. low conductivity b. low boiling point c. high malleability d. high solubility in water
Answers: 2
You know the right answer?
Determine the molar mass of (show your work) :
FeCl3•6H2O (note, this is all 1 molecule. Its an Fe...
Questions

Mathematics, 29.10.2020 20:20


Geography, 29.10.2020 20:20

Mathematics, 29.10.2020 20:20

Mathematics, 29.10.2020 20:20


History, 29.10.2020 20:20

Arts, 29.10.2020 20:20


History, 29.10.2020 20:20

Mathematics, 29.10.2020 20:20

Spanish, 29.10.2020 20:20


Mathematics, 29.10.2020 20:20



Mathematics, 29.10.2020 20:20

Mathematics, 29.10.2020 20:20


Mathematics, 29.10.2020 20:20