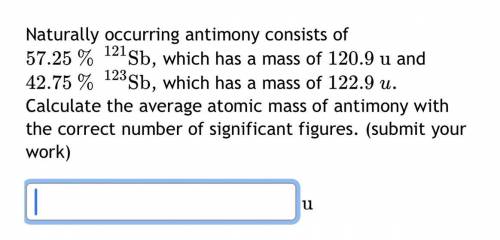
Naturally occurring antimony consists of
57.25
%
121
Sb
57.25
...

Naturally occurring antimony consists of
57.25
%
121
Sb
57.25
%
121
Sb
, which has a mass of
120.9
u
120.9
u
and
42.75
%
123
Sb
42.75
%
123
Sb
, which has a mass of
122.9
u
122.9
u
. Calculate the average atomic mass of antimony with the correct number of significant figures.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 08:00
Will give ! what are the advantages and disadvantages of nuclear power? check all that apply. one advantage of nuclear energy is that it does not produce carbon dioxide emissions. storage of nuclear waste is a short-term problem associated with nuclear energy. the problem with uranium mining is that a large quantity of uranium must be extracted to meet energy needs because the energy release from uranium fission is so low. safe operation of a nuclear power plant can be jeopardized by a human mistake.
Answers: 1

Chemistry, 22.06.2019 22:00
Ill give u brainliest pls how is mass of carbon conserved during cellular respiration
Answers: 1

Chemistry, 22.06.2019 22:30
Which is a characteristic of the electron sea model for metallic bonding? molecular orbitals overlap to produce bands. electrons flow easily between metal nuclei. electrons are in fixed positions in the orbitals. atomic nuclei are arranged in an irregular pattern.
Answers: 3

Chemistry, 22.06.2019 23:00
What extra step distinguishes fermentation from glycolysis
Answers: 1
You know the right answer?
Questions








Mathematics, 11.03.2020 02:42


Geography, 11.03.2020 02:42




Mathematics, 11.03.2020 02:42

Computers and Technology, 11.03.2020 02:42

Mathematics, 11.03.2020 02:42




Biology, 11.03.2020 02:42



