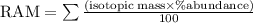Naturally occurring Indium has two isotopes.
4.28
%
4.28
%
of the atoms ar...

Chemistry, 13.02.2022 05:50 starfox5454
Naturally occurring Indium has two isotopes.
4.28
%
4.28
%
of the atoms are
Indium
−
113
Indium
-
113
(
113
In
113
In
) with a mass of
112.9
u
112.9
u
and
95.72
%
95.72
%
of the atoms are
Indium
−
115
Indium
-
115
(
115
In
115
In
) with a mass of
114.9
u
114.9
u
. Calculate the average atomic mass of Indium with the correct number of significant figures.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:40
How many electrons does silver have to give up in order to achieve a sido noble gas electron configuration
Answers: 1

Chemistry, 22.06.2019 08:20
What is the formula for the compound dinitrogen pentoxide? a. n4o5 b. n5o4 c. n4o6 d. n5o2 e. n2o5
Answers: 3

Chemistry, 22.06.2019 20:20
Which symbol can be used to indicate the pressure at which a chemical reaction is carried out? 25°c 2 atm pa
Answers: 2

You know the right answer?
Questions

Health, 08.07.2019 06:00

Physics, 08.07.2019 06:00


Mathematics, 08.07.2019 06:00


Physics, 08.07.2019 06:00

Mathematics, 08.07.2019 06:00

Mathematics, 08.07.2019 06:00






Biology, 08.07.2019 06:00



History, 08.07.2019 06:00