
Chemistry, 13.02.2022 04:40 DesireeGuzman5213
6) The average atomic mass of boron, B, is 10.811 amu. The natural abundance of 10B is 19.7% and it’s exact mass is 10.012 amu. Calculate the exact mass of the second isotope of boron.
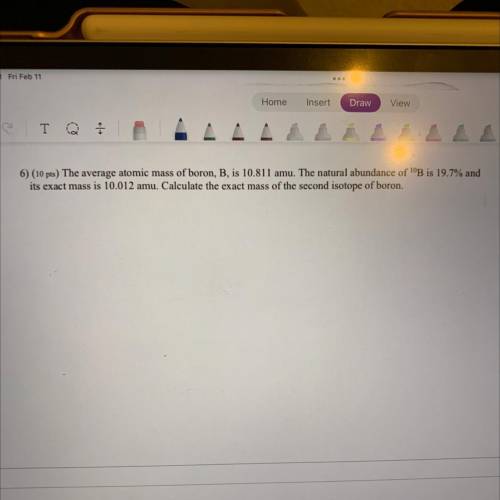

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 01:30
Arollercoaster car at the top of a hill has potential energy kinetic energy chemical energy light energy
Answers: 1

Chemistry, 23.06.2019 00:30
Maya wrote if you step to describe how carbon circulates between the atmosphere and living organisms
Answers: 1

Chemistry, 23.06.2019 11:30
If a refrigerator is a heat pump that follows the first law of thermodynamics, how much heat was removed from food inside of the refrigerator if it released 300j of energy to the room?unit:
Answers: 1

Chemistry, 23.06.2019 13:20
Aluminum reacts with sulfuric acid to produce aluminum sulfate and hydrogen gas. how many grams of aluminum sulfate would be formed if 250 g h 2 so 4 completely reacted with aluminum? 2al( s ) + 3h 2 so 4 ( aq ) ? al 2 (so 4 ) 3 ( aq ) + 3h 2 ( g )
Answers: 1
You know the right answer?
6) The average atomic mass of boron, B, is 10.811 amu. The natural abundance of 10B is 19.7% and it’...
Questions


Arts, 29.09.2021 01:00




Mathematics, 29.09.2021 01:00

Mathematics, 29.09.2021 01:00

Mathematics, 29.09.2021 01:00


Mathematics, 29.09.2021 01:00


Geography, 29.09.2021 01:00




Arts, 29.09.2021 01:00

Mathematics, 29.09.2021 01:00


Chemistry, 29.09.2021 01:00



