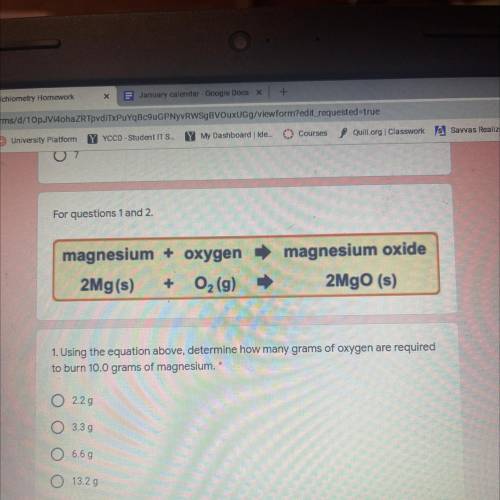
Magnesium + oxygen
2Mg(s) + O2 (9)
magnesium oxide
2MgO (s)
1. Using the equatio...

Chemistry, 11.02.2022 14:00 kayranicole1
Magnesium + oxygen
2Mg(s) + O2 (9)
magnesium oxide
2MgO (s)
1. Using the equation above, determine how many grams of oxygen are required
to burn 10.0 grams of magnesium.
2.29
O 3.39
6.69
Ο Ο
13.29

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 10:40
Ammonia and oxygen react to form nitrogen monoxide and water, like this: 4nh3 (g) + 5o2 (g) → 4no (g) + 6h2o (g) also, a chemist finds that at a certain temperature the equilibrium mixture of ammonia, oxygen, nitrogen monoxide, and water has the following composition: compound pressure at equilibrium nh3 65.1atm o2 31.3atm no 62.7atm h2o 65.8atm compound pressure at equilibrium nh3 65.3 atm o2 7.79 atm no 12.1 atm h2o 65.8 atm calculate the value of the equilibrium constant kp for this reaction. round your answer to 2 significant
Answers: 2

Chemistry, 22.06.2019 12:30
When a scientific theory has been tested and proved by the scientific community, it becomes a law
Answers: 2

Chemistry, 23.06.2019 01:30
How does the attraction between particles affect the ability of a solvent to dissolve in a substance
Answers: 1

Chemistry, 23.06.2019 06:00
Jenny wants to test the electrical conductivity of two substances dissolved in water. she is preparing the containers for the experiment. which factor is most important for her to control?
Answers: 1
You know the right answer?
Questions






Mathematics, 04.05.2021 17:50




Advanced Placement (AP), 04.05.2021 17:50




Computers and Technology, 04.05.2021 17:50


Mathematics, 04.05.2021 17:50


English, 04.05.2021 17:50




