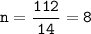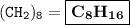Chemistry, 08.02.2022 14:00 tatilynnsoto17
A compound with the empirical formula CH2 was found to have a molar mass of approximately 112 g. Write the molecular formula of the compound.
2Points
Show all your work. Please use correct formatting for subscripts and exponents. The math formula editor makes it easier to show work.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 13:10
Cobalt-60 is an artificial radioisotope that is produced in a nuclear reactor and is used as a gamma-ray source in the treatment of certain types of cancer. if the wavelength of the gamma radiation from a cobalt-60 source is 1.00 × 10-3 nm, calculate the energy of a photon of this radiation.
Answers: 2

Chemistry, 22.06.2019 05:00
When you mate two plants together the terms is called? answer it fast as possible plz! i have a test tomorrow!
Answers: 1

Chemistry, 22.06.2019 15:30
Why does earth rotate? because earth is formed from cold gases collapsing due to gravity because the matter in the nebula that formed earth was spinning because earth forms more than 99% of the mass of the solar system because the hydrogen atoms inside the nebula fused to form helium
Answers: 1

Chemistry, 22.06.2019 20:00
What is the molar mass of the anhydrous compound? answer using four significant figures. 36.02 g/mol 120.15 g/mol 156.12 g/mol
Answers: 1
You know the right answer?
A compound with the empirical formula CH2 was found to have a molar mass of approximately 112 g. Wri...
Questions


Mathematics, 24.07.2019 13:40

Biology, 24.07.2019 13:40

Biology, 24.07.2019 13:40

Biology, 24.07.2019 13:40


Biology, 24.07.2019 13:40


Biology, 24.07.2019 13:40

History, 24.07.2019 13:40


Biology, 24.07.2019 13:40

Biology, 24.07.2019 13:40


Advanced Placement (AP), 24.07.2019 13:40

Biology, 24.07.2019 13:40

History, 24.07.2019 13:40


Mathematics, 24.07.2019 13:40

History, 24.07.2019 13:40