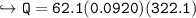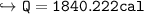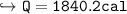Charlie the Chemist heats a 62.51 g piece of copper from 22.9°C to 345.0°C. Calculate the amount
of heat absorbed by the piece of metal (with correct significant figures and sign). Show your work,
Note: The specific heat of copper is 0.0920 cal(g°C), and cal is the abbreviation for calorie.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:30
What is the most stable monatomic ion formed from nitrogen
Answers: 2

Chemistry, 22.06.2019 10:00
3. how much energy in joules is required to evaporate .0005 kg of liquid ammonia to vapor at the same temperature? 4. how much energy ( in megajoules ) is given up by .75 kg of water at 0c when it freezes to form ice at 0c? 5. explain how heat works between and at critical temperatures?
Answers: 2

Chemistry, 22.06.2019 10:30
Rocks, as they are compressed, begin forming mountains above the earth's surface when two continental plates converge. the continental crust increases in depth as the mountains grow above. the himalayan mountains formed at a convergent plate boundary in this manner. the rocks are smashed together causing them to due to the intense heat and pressure from the colliding plates and eventually forming rock. a) melt; igneous b) layer; sedimentary c) recrystallize; metamorphic d) melt into the earth's interior; metamorphic
Answers: 1

Chemistry, 22.06.2019 13:30
Apush or pull that moves or changes and object when to objects touch
Answers: 2
You know the right answer?
Charlie the Chemist heats a 62.51 g piece of copper from 22.9°C to 345.0°C. Calculate the amount
o...
Questions




Mathematics, 01.07.2021 05:30


Medicine, 01.07.2021 05:30

Mathematics, 01.07.2021 05:30

Mathematics, 01.07.2021 05:30



History, 01.07.2021 05:30

Mathematics, 01.07.2021 05:30




Mathematics, 01.07.2021 05:30


Mathematics, 01.07.2021 05:30

History, 01.07.2021 05:30

Mathematics, 01.07.2021 05:40