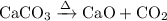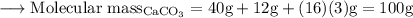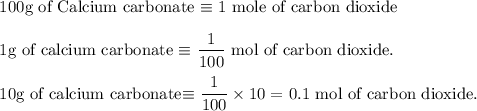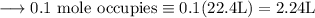Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 02:40
Consider the nuclear equation below. 239/94 pu—-> x+ 4/2 he. what is x?
Answers: 2

Chemistry, 22.06.2019 08:30
Sally is making a model of a magnesium atom with an atomic mass number of 24 for her chemistry class. she has foam balls for the protons, neutrons, and electrons. she has added 6 neutrons to her model so far. how many more neutrons does she need to add to complete her neutral atom of magnesium?
Answers: 1

Chemistry, 23.06.2019 03:00
What happens in the particles of a gas when the gas is compressed
Answers: 1

Chemistry, 23.06.2019 04:00
Why must humans find substitutes for many minerals found on earth? (a) form at an extremely slow rate (b) controlled by other countries (c) too deep in the earth to collect
Answers: 1
You know the right answer?
Write an equation for the thermal decomposition of CaCO3. Determine the volume of CO2 measured at s...
Questions



Mathematics, 30.12.2019 22:31

Biology, 30.12.2019 22:31

Business, 30.12.2019 22:31



English, 30.12.2019 22:31



Mathematics, 30.12.2019 22:31

English, 30.12.2019 22:31

Biology, 30.12.2019 22:31

English, 30.12.2019 22:31

Mathematics, 30.12.2019 22:31