
Chemistry, 06.02.2022 01:00 lillierudloff2558
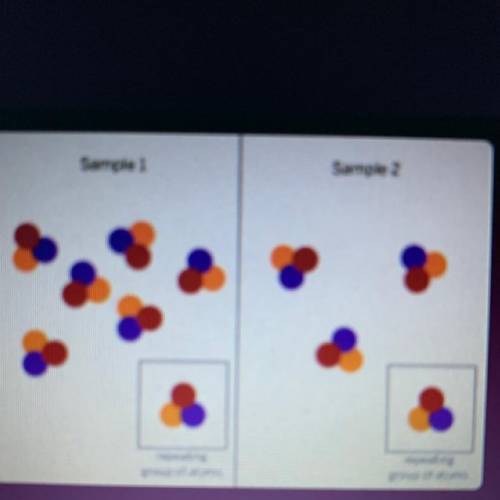
The diagram above shows the repeating groups of atoms that make
up two samples. Both samples are red powdery solids at room
temperature. Will the other properties of the two samples be the
same or different? (Examples of properties are smell, color, and the
temperature at which a substance melts.)
The other properties
will be same because
the repeating groups
of atoms that make up
the two samples are
the same
The other properties
will be the same
because both samples
are powdery solids at
room temperature
The other properties - The other properties
will be different
will be the same
because there are because both
more repeating groups samples are red at
LOlerome in Sample 1.
room temperature.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:30
Melting and boiling are endothermic processes. this means that these processes absorb energy from their surroundings in order to occur. use this information and the data you collected in the phase change gizmo to describe what happens to the temperature of water when you boil it, then explain why this result occurs.
Answers: 1

Chemistry, 22.06.2019 09:00
The diagram below shows a cell placed in a solution.a cell is shown placed inside a beaker. it is labeled cell. the solution inside the beaker is labeled 40% salt solution and the solution inside the cell is labeled 20% salt solution.only water is allowed to move in and out of the cell. what will most likely happen to the cell? it will expand as water moves out of it. it will shrink as water moves out of it.it will expand as water moves into it. it will shrink as water moves into it.
Answers: 2

Chemistry, 22.06.2019 20:30
We are hoping to create 5.72 grams of glucose. the plant was given 4.75 liters of co2 and 2.81 g of h20. which reactant was the limiting reagent? how much excess mass did we have of the other reactant?
Answers: 3

Chemistry, 22.06.2019 21:00
Acandle’s wick is the fabric string that holds the flame, and it burns down at a constant slow pace when the candle is lit. the wick is usually surrounded by wax. which is the most important property of covalent compounds that makes them useful for making candle wax? a low boiling point a low melting point a high boiling point a high melting point
Answers: 1
You know the right answer?
The diagram above shows the repeating groups of atoms that make
up two samples. Both samples are r...
Questions

Biology, 22.07.2019 19:00

Biology, 22.07.2019 19:00



Computers and Technology, 22.07.2019 19:00

Computers and Technology, 22.07.2019 19:00

Computers and Technology, 22.07.2019 19:00

Computers and Technology, 22.07.2019 19:00


Mathematics, 22.07.2019 19:00





Chemistry, 22.07.2019 19:00


Social Studies, 22.07.2019 19:00

Mathematics, 22.07.2019 19:00

Social Studies, 22.07.2019 19:00

Social Studies, 22.07.2019 19:00




