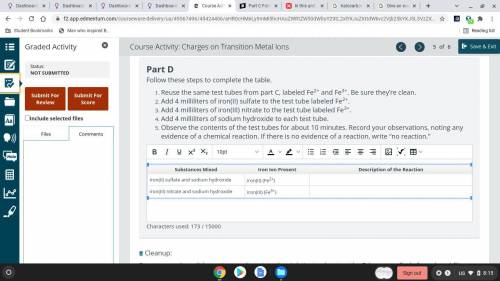
Part D
Follow these steps to complete the table.
Reuse the same test tubes from part C...

Part D
Follow these steps to complete the table.
Reuse the same test tubes from part C, labeled Fe2+ and Fe3+. Be sure they’re clean.
Add 4 milliliters of iron(II) sulfate to the test tube labeled Fe2+.
Add 4 milliliters of iron(III) nitrate to the test tube labeled Fe3+.
Add 4 milliliters of sodium hydroxide to each test tube.
Observe the contents of the test tubes for about 10 minutes. Record your observations, noting any evidence of a chemical reaction. If there is no evidence of a reaction, write “no reaction.”

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 11:30
If blood contains 150g of hemoglobin per liter of blood, how much hemoglobin would be contained in 10 ml of blood
Answers: 2

Chemistry, 22.06.2019 23:10
Using the periodic table, complete the following. element: hydrogen symbol: h₂ molecular weight: g mass of one mole: g/mol
Answers: 3

Chemistry, 23.06.2019 00:00
How many moles of water are created if 3 moles of hydrogen react completely with excess oxygen?
Answers: 3

Chemistry, 23.06.2019 00:20
What type of context clue you understand the meaning of quandary?
Answers: 3
You know the right answer?
Questions

English, 12.02.2021 21:00


History, 12.02.2021 21:00

French, 12.02.2021 21:00


Advanced Placement (AP), 12.02.2021 21:00

Mathematics, 12.02.2021 21:00

Mathematics, 12.02.2021 21:00

Advanced Placement (AP), 12.02.2021 21:00


Mathematics, 12.02.2021 21:00

History, 12.02.2021 21:00

Chemistry, 12.02.2021 21:00

Mathematics, 12.02.2021 21:00


Spanish, 12.02.2021 21:00


Social Studies, 12.02.2021 21:00

Mathematics, 12.02.2021 21:00



