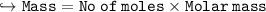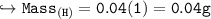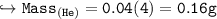Compare the masses of a one-liter sample of hydrogen and a one-liter sample of helium gas, each at 25°C and 5.0 atm pressure.
I will mark brainliest if right!!
A) the helium gas has twice the mass of the hydrogen gas
B) the helium gas has four times the mass of the hydrogen gas
C) the hydrogen gas has twice the mass of the helium gas
D) the hydrogen gas has four times the mass of the helium gas
E) the mass of the hydrogen gas equals the mass of the helium gas

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:30
Jessica is traveling from miami, florida, to chicago, illinois. using the map, tell one way the land will change during the second half of her trip.
Answers: 1

Chemistry, 22.06.2019 01:30
(apex) when a cup of water is dropped, as the cup falls, the water in the cup falls out true or false?
Answers: 1

Chemistry, 22.06.2019 19:30
Describe the forces both attractive and repulsive that occur as two atoms move closer together.
Answers: 1

Chemistry, 22.06.2019 20:30
What is a difference between a mixture of elements and a mixture of compounds
Answers: 1
You know the right answer?
Compare the masses of a one-liter sample of hydrogen and a one-liter sample of helium gas, each at 2...
Questions


Physics, 18.12.2019 18:31

Mathematics, 18.12.2019 18:31

History, 18.12.2019 18:31


History, 18.12.2019 18:31

Mathematics, 18.12.2019 18:31

Biology, 18.12.2019 18:31

Mathematics, 18.12.2019 18:31

Social Studies, 18.12.2019 18:31


Geography, 18.12.2019 18:31



Mathematics, 18.12.2019 18:31

Health, 18.12.2019 18:31