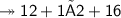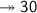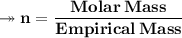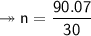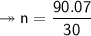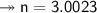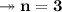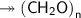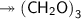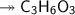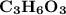Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:50
What are transitions between a liquid and gas called? identify which way they are transitioning
Answers: 2

Chemistry, 22.06.2019 10:50
Determine the empirical formula for succinic acid that is composed of 40.60% carbon, 5.18% hydrogen, and 54.22% oxygen.
Answers: 1

Chemistry, 22.06.2019 12:00
An atom of which element reacts with an atom of hydrogen to form a bond with the greatest degree of polarity ?
Answers: 1

Chemistry, 22.06.2019 15:00
Which of the following is the correct formula for copper (i) sulfate trihydrate? cuso4 · 3h2o cuso4(h2o)3 cu2so4(h2o)3 cu2so4 · 3h2o
Answers: 1
You know the right answer?
Molar mass of a compound is 90.07 g/mol. Determine its molecular formula if its empirical
formula...
Questions

Mathematics, 19.11.2020 02:40


Mathematics, 19.11.2020 02:40




Mathematics, 19.11.2020 02:40


Mathematics, 19.11.2020 02:40

History, 19.11.2020 02:40

History, 19.11.2020 02:40

Mathematics, 19.11.2020 02:40


Health, 19.11.2020 02:40

English, 19.11.2020 02:40

SAT, 19.11.2020 02:40



Mathematics, 19.11.2020 02:40

Business, 19.11.2020 02:40

 ☀️
☀️
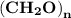
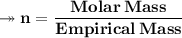
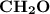 –
–