
Chemistry, 31.01.2022 07:10 harleypage308
Please help!
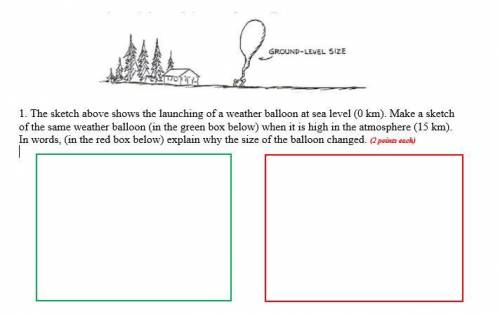
1. The sketch above shows the launching of a weather balloon at sea level (0 km). Make a sketch of the same weather balloon (in the green box below) when it is high in the atmosphere (15 km). In words, (in the red box below) explain why the size of the balloon changed
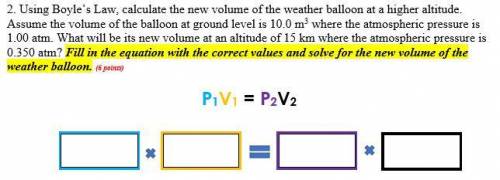
2. Using Boyle’s Law, calculate the new volume of the weather balloon at a higher altitude. Assume the volume of the balloon at ground level is 10.0 m3 where the atmospheric pressure is 1.00 atm. What will be its new volume at an altitude of 15 km where the atmospheric pressure is 0.350 atm? Fill in the equation with the correct values and solve for the new volume of the weather balloon.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 00:30
Elements that do not have full outer electron shells will donate, share, or take electrons from other atoms. choose the items that have the correct binary ionic formula.
Answers: 2

Chemistry, 22.06.2019 13:30
An animal cell loses the ability to convert energy stored in food to energy that the cell can use. which of the cell's organelles has stopped working? a.the mitochondria b.the nucleus c.the vacuoles d.the endoplasmic reticulum
Answers: 1

Chemistry, 22.06.2019 21:30
How can the periodic table be used to predict the behavior of elements?
Answers: 1

You know the right answer?
Please help!
1. The sketch above shows the launching of a weather balloon at sea level (0 km). Mak...
Questions


History, 01.03.2021 23:30

Business, 01.03.2021 23:30

Geography, 01.03.2021 23:30






History, 01.03.2021 23:30


Advanced Placement (AP), 01.03.2021 23:30

Mathematics, 01.03.2021 23:30

Physics, 01.03.2021 23:30








