
Chemistry, 24.01.2022 09:20 cguzman4993
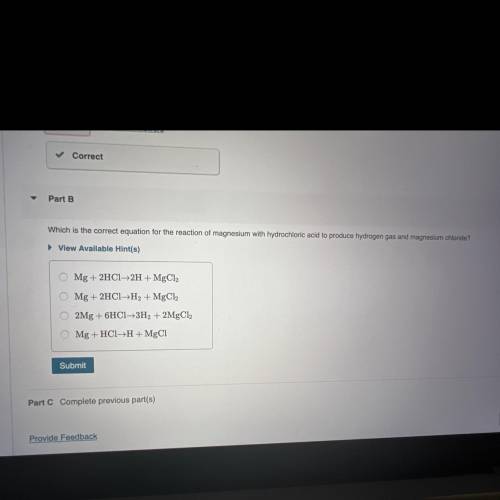
Which is the correct equation for the reaction of magnesium with hydrochloric acid to produce hydrogen gas and magnesium chloride?
View Available Hint(s)
O Mg + 2HCl -> 2H + MgCl2
O Mg + 2HCl -> H2 + MgCl2
O2Mg + 6HCl -> 3H2 + 2MgCl2
O Mg + HCl -> H+MgCl

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 02:40
The difference between the atomic number of an element and the element’s atomic mass is the number of ions.
Answers: 3

Chemistry, 22.06.2019 14:10
Aconcentrated solution of ammonia is 14.8m and has a density of 0.899g/l. what is the concentration of ammonia in this solution in weight percent (%w/w)?
Answers: 1

Chemistry, 22.06.2019 16:00
Which of the following is the correct definition of chemical energy? a. energy an object has because of its motion or position b. energy resulting from the flow of charged particles, such as electrons or ions c. energy produced from the splitting of atoms d. energy stored in chemical bonds of molecules
Answers: 1

Chemistry, 22.06.2019 17:20
The small bags of silica gel you often see in a new shoe box are placed there to control humidity. despite its name, silica gel is a solid. it is a chemically inert, highly porous, amorphous form of sio2. because water vapor readily adsorbs onto the surface of silica gel, it acts as a desiccant. despite not knowing mechanistic details of the adsorption of water onto silica gel, from the information provided you should be able to make an educated guess about the thermodynamic characteristics of the process. predict the signs for δg, δh, and δs for the adsorption of water.
Answers: 2
You know the right answer?
Which is the correct equation for the reaction of magnesium with hydrochloric acid to produce hydrog...
Questions


Mathematics, 24.05.2021 06:20



History, 24.05.2021 06:20




Biology, 24.05.2021 06:20

Mathematics, 24.05.2021 06:20




Mathematics, 24.05.2021 06:20








