HELP PLEASE
Use the following steps to balance the redox reaction below:
Mg + Au+ Mg2+ + Au<...

Chemistry, 23.01.2022 14:00 sarahgrindstaff123
HELP PLEASE
Use the following steps to balance the redox reaction below:
Mg + Au+ Mg2+ + Au
a. Write the oxidation and reduction half-reactions. Make sure each half-reaction is balanced for number of atoms
and charge. (3 points)
b. Multiply each half reaction by the correct number in order to balance charges for the two half reactions
c. Add the equations and simplify to get a balanced equation
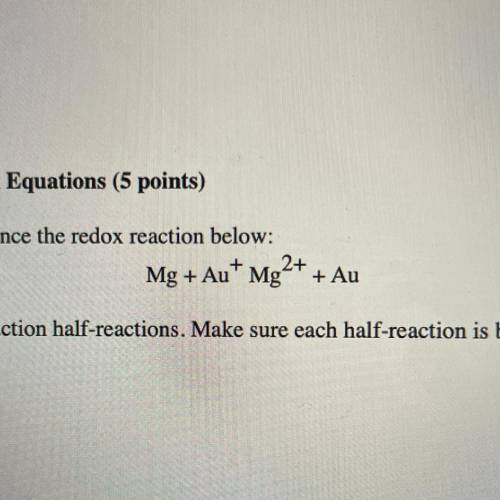

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 12:50
5. how can you decrease the pressure of a gas in a container without changing the volume of the gas?
Answers: 1

Chemistry, 22.06.2019 13:00
What is the mass of 2.00 l of an intravenous glucose solution with a density of 1.15 g/ml?
Answers: 2

Chemistry, 22.06.2019 18:00
Which statement best describes the he properties of iconic compounds ?
Answers: 1

Chemistry, 22.06.2019 23:00
Consider the reaction: 2al(s) + fe2o3(s) → al2o3(s) + 2fe(s) the δhf for fe2o3(s) = -824.3 kj/mole. the δhf for al2o3(s) = -1675.7 kj/mole. finish the equation. δhrxn = [(1)( kj/mole) + (2)( kj/mole)] - [(1)( kj/mole) + (2) ( kj/mole)]
Answers: 1
You know the right answer?
Questions


Spanish, 01.02.2021 06:50


Mathematics, 01.02.2021 06:50

Mathematics, 01.02.2021 06:50

English, 01.02.2021 06:50









Mathematics, 01.02.2021 06:50


Geography, 01.02.2021 06:50

Mathematics, 01.02.2021 06:50

Social Studies, 01.02.2021 06:50



