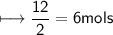Chemistry, 21.01.2022 14:00 melvina13bday
You are asked to make 12 moles of iron(Fe) from iron oxide and carbon monoxide.
Fe2O3(s) + 3CO(g)→2Fe(l) + 3CO2(g)
Approximately how many moles of iron oxide(Fe2O3) is used?

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 18:00
How many moles of oxygen gas are produced from the decomposition of six moles of potassium chlorate
Answers: 3

Chemistry, 23.06.2019 08:30
Of the following elements, which is the least reactive? a. c b. h c. li d. he
Answers: 1

Chemistry, 23.06.2019 09:30
Which of the following is not a characteristic of a hydrogen bond? 1. it is responsible for the unusual physical properties of water. 2. it is weaker than a covalent bond. 3. it is stronger than other dipole-dipole interactions. 4. it can occur when hydrogen is covalently bound to very electronegative elements liks f, cl, br and i.
Answers: 1
You know the right answer?
You are asked to make 12 moles of iron(Fe) from iron oxide and carbon monoxide.
Fe2O3(s) + 3CO(g)→...
Questions


English, 02.07.2019 20:20

Mathematics, 02.07.2019 20:20


Health, 02.07.2019 20:20


Social Studies, 02.07.2019 20:20


Mathematics, 02.07.2019 20:20

Mathematics, 02.07.2019 20:20

French, 02.07.2019 20:20

Spanish, 02.07.2019 20:20



Mathematics, 02.07.2019 20:20

History, 02.07.2019 20:20



Computers and Technology, 02.07.2019 20:20