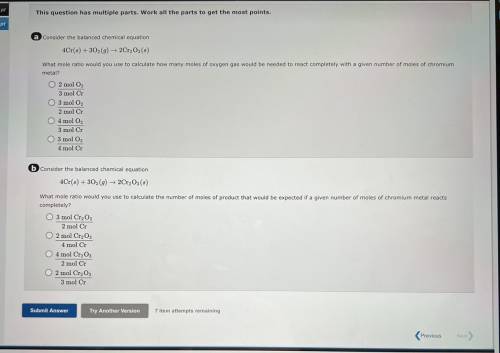
A consider the balanced chemical equation
4Cr(6) + 30(9) 2Cr, Os(8)
What mole ratio would yo...

Chemistry, 21.01.2022 01:00 aliciaa101
A consider the balanced chemical equation
4Cr(6) + 30(9) 2Cr, Os(8)
What mole ratio would you use to calculate how many moles of oxygen gas would be needed to react completely with a given number of moles of chromium
metal?
O 2 mol O,
3 mol Cr
O 3 mol O2
2 mol Cr
04 mol O
3 mol C
O 3 mol O2
4 mol Cr
6 Consider the balanced chemical equation
4Cr(6) +302 (9) ► 2Cr, Os()
What mole ratio would you use to calculate the number of moles of product that would be expected if a given number of moles of chromium metal reacts
completely?
O 3 mol Cr2O3
2 mol C
O 2 mol CrgO;
4 mol Cr
O 4 mol Cr2O3
2 mol Cr
O 2 mol Cryo,
3 mol Cr

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 23:30
Write a paragraph that provides examples of each stage of volcanic activity, a description of the volcano, and facts about each stage.
Answers: 1

Chemistry, 22.06.2019 04:00
4. absorption has the highest risk of overdose due to increased potency. a. rectal b. oral c. transdermal d. intranasal
Answers: 2

Chemistry, 22.06.2019 05:30
Transportation is the largest single source of air pollution in the united states. air pollution can harm the environment and human health. which technology could offer a solution to this problem? mufflers that reduce noise motors that run on electricity tires that improve gas mileage
Answers: 3

Chemistry, 23.06.2019 14:00
How many moles of oxygens atoms are present in 5.00 mol of diphosphorus of fe2(so4)3
Answers: 2
You know the right answer?
Questions

Mathematics, 16.06.2021 17:00


Social Studies, 16.06.2021 17:00





Social Studies, 16.06.2021 17:10

Health, 16.06.2021 17:10

Mathematics, 16.06.2021 17:10

Mathematics, 16.06.2021 17:10




History, 16.06.2021 17:10

Mathematics, 16.06.2021 17:10



Physics, 16.06.2021 17:10



