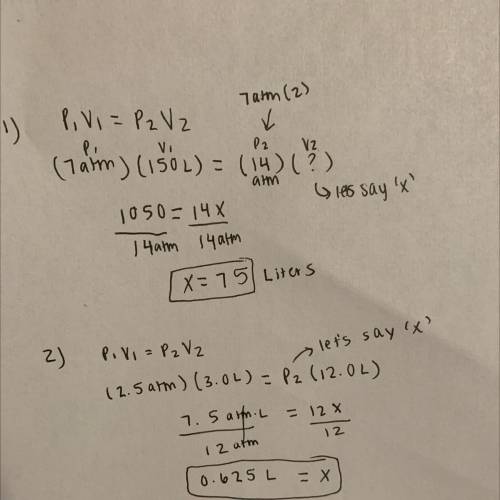Chemistry, 16.01.2022 07:50 sullivanjakob
1.A gas sample has a volume of 150. L when the pressure is 7.00 atm. If the temperature and amount of gas remains constant, what volume will the gas sample occupy at a pressure is doubled?
2. A helium gas in a balloon occupies 3.0 L at 2.5 atm. At what pressure will it point occupy 12 L assuming the mass and the temperature are constant?
Please answer correctly with evidence both parts

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 23:30
Calculate the expected ph values of the buffer systems from the experiments (a,b,c,d), using the henderson- hasselbalch equation, ph-pka+log[a-]/[ha]. use for pka values carbonic acid= 6.37, and acetic acid= 4.75.
Answers: 2

Chemistry, 22.06.2019 13:50
Read the chemical equation. 2c2h2 + 5o2 → 4co2 + 2h2o which of the following statements would be correct if one mole of c2h2 was used in this reaction? one mole of oxygen was used in this reaction. five moles of oxygen were used in this reaction. four moles of carbon dioxide were produced from this reaction. two moles of carbon dioxide were produced from this reaction.
Answers: 3


Chemistry, 23.06.2019 02:00
Which best describes the present-day universe? opaque, expanding very slowly, stars produce heavy elements transparent, expanding at an accelerated rate, stars produce heavy elements opaque, expanding at an accelerated rate, stars produce only hydrogen and helium transparent, expanding very slowly, stars produce only hydrogen and helium
Answers: 1
You know the right answer?
1.A gas sample has a volume of 150. L when the pressure is 7.00 atm. If the temperature and amount o...
Questions


Mathematics, 27.09.2021 14:10


Mathematics, 27.09.2021 14:10


Biology, 27.09.2021 14:10

Health, 27.09.2021 14:10

Biology, 27.09.2021 14:10

Biology, 27.09.2021 14:10

Mathematics, 27.09.2021 14:10

History, 27.09.2021 14:10

Spanish, 27.09.2021 14:10


Mathematics, 27.09.2021 14:10


Biology, 27.09.2021 14:10