
Chemistry, 27.12.2021 03:20 meababy2009ow9ewa
Burning 1.6 grams of substance M requires 6.4 grams of oxygen to produce carbon dioxide (CO2) and water (H2O) according to the mass ratio:
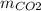 :
: 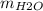 = 11 : 9
= 11 : 9
Calculate the mass of CO2 and H2O produced.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 19:00
Which of the following best explains why the end of a spoon sticking out of a cup of hot water also gets hot? question 7 options: the heat from the hot water is conducted through the spoon handle the hot water heats the air surrounding the upper part of the spoon. the hot water causes a physical change in the spoon handle. the hot water causes a chemical reaction to take place in the spoon.
Answers: 2

Chemistry, 21.06.2019 20:50
Choose all that apply. when creating a graph, you should: determine the x- and y- variables label the scale on the x- and y- axes plot the data points draw a line of best fit to represent the data trend
Answers: 1

Chemistry, 21.06.2019 22:00
Which solution is an example of a nonelectrolyte? a. iodine in hexane b. sodium nitrate in waterc. acetic acid in waterd. hydrogen chloride in water
Answers: 2

Chemistry, 22.06.2019 00:00
Substance x has a fixed volume, and the attraction between its particles is strong .substance y had widely spread out particles and can be compressed what can most likely be concluded about these substances
Answers: 2
You know the right answer?
Burning 1.6 grams of substance M requires 6.4 grams of oxygen to produce carbon dioxide (CO2) and wa...
Questions

Mathematics, 04.06.2020 19:11

Biology, 04.06.2020 19:11






Mathematics, 04.06.2020 19:11



English, 04.06.2020 19:11




English, 04.06.2020 19:12




Physics, 04.06.2020 19:12

Mathematics, 04.06.2020 19:12



