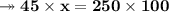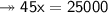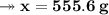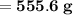Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 01:20
Match the acid base pairs by arranging the acid name with the conjugate base formula. hydrogen carbonate hydrogen phosphate carbonic acid read water sulfuric acid phosphoric acid a. co32- b. hso4- c. hco3- d. po43- e. h2po4- f. oh-
Answers: 1


Chemistry, 22.06.2019 15:00
What is the most important factor in determining climates.
Answers: 1

Chemistry, 22.06.2019 20:30
The activation energy for the reaction no2(g)+co2(g)⟶no(g)+co(g) is ea = 300 kj/mol and the change in enthalpy for the reaction is δh = -100 kj/mol . what is the activation energy for the reverse reaction?
Answers: 3
You know the right answer?
Knowing that the solubility of a salt at 80°C is 45g/100 g of H, O, calculate the mass of water requ...
Questions

History, 31.10.2019 23:31


Mathematics, 31.10.2019 23:31

Chemistry, 31.10.2019 23:31



English, 31.10.2019 23:31



Mathematics, 31.10.2019 23:31

Mathematics, 31.10.2019 23:31

Mathematics, 31.10.2019 23:31

Geography, 31.10.2019 23:31

Chemistry, 31.10.2019 23:31


English, 31.10.2019 23:31

Physics, 31.10.2019 23:31

Mathematics, 31.10.2019 23:31

History, 31.10.2019 23:31

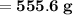 .
. 45g of salt
45g of salt  100g of H₂O
100g of H₂O