
Chemistry, 20.12.2021 22:10 jesuscruzm2020
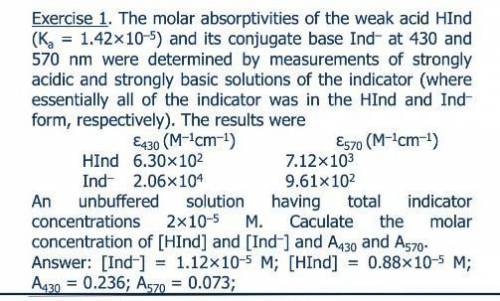
The molar absorptivities at 430 and 570 nm of the weak acid HIn (Ka = 1.42 ? 10-5) and its conjugate base In- were determined by measurements of strongly acidic and strongly basic solutions of the indicator. Under this conditions, essentially all of the indicator was in the HIn and In- form, respectively. The molar ab-sorptivities of HIn and In- at 430 nm and 570 nm were 6.30 ? 102 and 7.12 ? 103, and 2.06 ? 104 and 9.61 ? 102, respectively. Calculate absorbance data for unbuffered solutions that have total indicator concentrations ranging from 2 ? 10-5 to 16 ? 10-5 M.

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 13:50
How does the motion of particles in a gas change as the gas cools
Answers: 2


Chemistry, 22.06.2019 19:30
What is the area in square meters of 448 g ai foil that has a thickness of 23921 nm? the density is 2.70 g/cm
Answers: 3
You know the right answer?
The molar absorptivities at 430 and 570 nm of the weak acid HIn (Ka = 1.42 ? 10-5) and its conjugate...
Questions

Mathematics, 02.12.2019 15:31



History, 02.12.2019 15:31


History, 02.12.2019 15:31

Mathematics, 02.12.2019 15:31


Physics, 02.12.2019 15:31

Arts, 02.12.2019 15:31

Mathematics, 02.12.2019 15:31


Social Studies, 02.12.2019 15:31

History, 02.12.2019 15:31


English, 02.12.2019 15:31


Biology, 02.12.2019 15:31




