
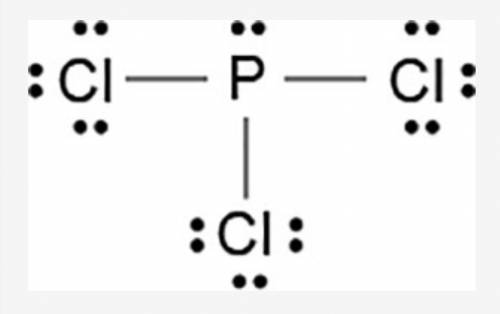
The Lewis dot model of a molecule is shown.
A visual diagram of a PCl3 molecule is shown. Phosphorous is the central atom with a horizontal line connecting to each of the three Chlorine atoms around it. Phosphorous has a pair of dots on it. Each of the three chlorine atoms have a pair of three dots on it.
Based on the model, which of the following is true?
The electronegativity difference between phosphorous and chlorine is greater than 1.7.
Each chlorine has three non-bonded pairs and one bonded pair of electrons.
Phosphorous has three non-bonded pairs and one bonded pair of electrons.
Phosphorous has three valence electrons in the outermost energy level.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 14:00
650.j is the same amount of energy as? 2720cal1550cal650.cal2.72cal
Answers: 2

Chemistry, 22.06.2019 17:50
Cryolite, na3alf6(s), an ore used in the production of aluminum, can be synthesized using aluminum oxide. start this question by first balance the chemical equation.1.) balance the equation: - alo3(s)+naoh(l)+hf(> na3alf6+h2o(g). 2.) if 17.5 kilograms of al2o3(s), 51.4 kilograms of naoh(l), and 51.4 kilograms of hf(g) react completely, how many kilograms of cryolite will be produced? 3.)which reactants will be in excess, (al2o3, naoh, or hf) 4.)what is the total mass of the excess reactants left over after the reaction is complete in kg?
Answers: 2

Chemistry, 22.06.2019 23:30
Why do oxygen have a strong attractive force for electrons
Answers: 2

You know the right answer?
The Lewis dot model of a molecule is shown.
A visual diagram of a PCl3 molecule is shown. Phosphor...
Questions

History, 17.07.2019 06:30

Business, 17.07.2019 06:30




Biology, 17.07.2019 06:30


Physics, 17.07.2019 06:30

Mathematics, 17.07.2019 06:30






Mathematics, 17.07.2019 06:30

History, 17.07.2019 06:30




History, 17.07.2019 06:30



