
Chemistry, 16.12.2021 19:50 shorty178658
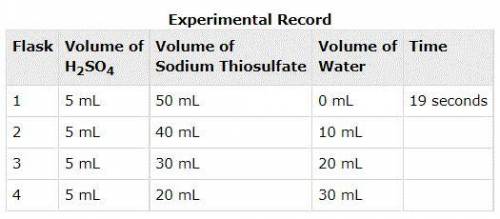
In an experiment, sulfuric acid reacted with different volumes of sodium thiosulfate in water. A yellow precipitate was formed during the reaction. A cross drawn at the base of each flask became gradually invisible due to the formation of this yellow precipitate. The time taken for the cross to become invisible was recorded. A partial record of the experiment is shown. Based on your knowledge of factors that affect the rates of chemical reactions, predict the trend in the last column of the experimental record. Use complete sentences to explain the trend you predicted. You do not have to determine exact values for time; just describe the trend you would expect (increase or decrease) and why it occurs.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 08:30
Joan writes four numbers on the board in standard form, and then she writes their scientific notation
Answers: 1


Chemistry, 22.06.2019 11:00
The twister and runaway train are two coasters at the same amusement park. both coasters start at the same height. the coaster for the twister is twice the mass of the coaster for the runaway train. which roller coaster has greater gravitational potential energy at the start of the ride?
Answers: 1

Chemistry, 22.06.2019 19:20
Anyone who's in connections academy chemistry b have the factors that affect the rate of a reaction portfolio already done?
Answers: 3
You know the right answer?
In an experiment, sulfuric acid reacted with different volumes of sodium thiosulfate in water. A yel...
Questions


Mathematics, 09.02.2021 20:00

Physics, 09.02.2021 20:00



Mathematics, 09.02.2021 20:00

Computers and Technology, 09.02.2021 20:00

Mathematics, 09.02.2021 20:00

Mathematics, 09.02.2021 20:00

English, 09.02.2021 20:00

Mathematics, 09.02.2021 20:00




Mathematics, 09.02.2021 20:00





English, 09.02.2021 20:00



