
Chemistry, 15.12.2021 03:50 kingbob101
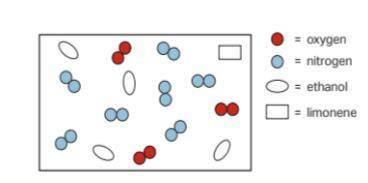
The partial pressure of the nitrogen gas in the sample is 0.70 atm.
Calculate the total pressure of the gas sample.
Calculate the partial pressure of the ethanol in the sample.
PLEASE HELP THIS IS DUE TODAY AND I REALLY NEED HELP.
Giving brainliest.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 21:40
During trial 2, what allowed you to determine that aluminum was the limiting reactant? check all that apply. all of the copper dissolved. all of the aluminum dissolved. the solution turned clear. the number of grams of copper(ii) chloride used in the reaction was greater than the number of grams of aluminum. the molar ratio of copper(ii) chloride to aluminum was greater than 3: 2, the equation’s molar ratio.
Answers: 2

Chemistry, 22.06.2019 06:00
Compare and contrast physical changes with chemical changes.
Answers: 3

Chemistry, 22.06.2019 21:50
Liquid from a brewery fermentation contains 10% ethanol and 90% water. part of the fermentation product (50,000 kg/h) is pumped to a distillation column on the factory site. under current operating conditions, a distillate of 45% ethanol and 55% water is produced from the top of the column at a rate of one-tenth that of the feed. what is the composition of the waste "bottoms" from the still?
Answers: 2

Chemistry, 23.06.2019 02:00
Why does ammonia, nh3, behave as a base when it reacts with an acid? z
Answers: 2
You know the right answer?
The partial pressure of the nitrogen gas in the sample is 0.70 atm.
Calculate the total pressure o...
Questions

History, 06.07.2019 11:30


History, 06.07.2019 11:30






History, 06.07.2019 11:30


History, 06.07.2019 11:30

History, 06.07.2019 11:30



History, 06.07.2019 11:30


Mathematics, 06.07.2019 11:30



Mathematics, 06.07.2019 11:30



