Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 21:40
During trial 2, what allowed you to determine that aluminum was the limiting reactant? check all that apply. all of the copper dissolved. all of the aluminum dissolved. the solution turned clear. the number of grams of copper(ii) chloride used in the reaction was greater than the number of grams of aluminum. the molar ratio of copper(ii) chloride to aluminum was greater than 3: 2, the equation’s molar ratio.
Answers: 2

Chemistry, 21.06.2019 22:30
Agas at 155 kpa and standard temperature has an initial volume of 1.00 l. the pressure of the gas rises to 500 kpa as the temperature also rises to 135°c. what is the new volume? 2.16 l 0.463 l 0.207 l 4.82 l
Answers: 3

Chemistry, 22.06.2019 00:00
Several kinds of bears are found on earth. most bears are brown or black, but one type of bear, the polar bear, is white. what process led to this difference in fur color? explain your answer.
Answers: 1

Chemistry, 22.06.2019 02:50
The conventional equilibrium constant expression (kc) for the system below is: 2icl(s) ⇄ i2(s) + cl2(g) [cl2] ([i2] + [cl2])/2[icl] [i2][cl2]/[icl]2 none of the listed answers are correct [i2][cl2]/2[icl]
Answers: 2
You know the right answer?
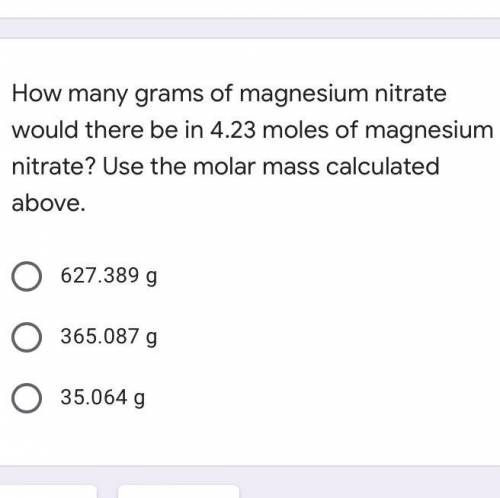
How many grams of magnesium nitrate would there be in 4.23 moles of magnesium nitrate?
A. 627.389...
Questions

Physics, 07.12.2020 14:00

English, 07.12.2020 14:00






History, 07.12.2020 14:00


Geography, 07.12.2020 14:00

Advanced Placement (AP), 07.12.2020 14:00

Mathematics, 07.12.2020 14:00




Mathematics, 07.12.2020 14:00

Mathematics, 07.12.2020 14:00

Biology, 07.12.2020 14:00