
Chemistry, 14.12.2021 21:30 slawson4328
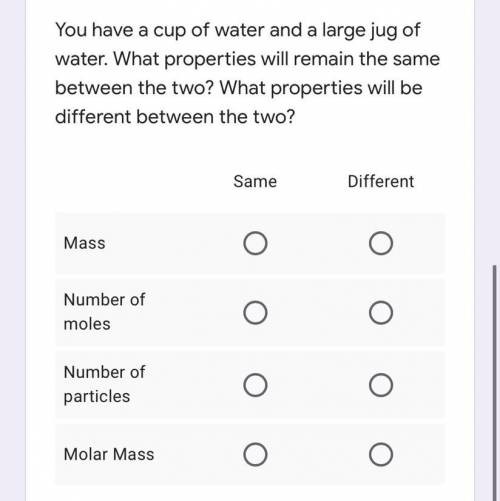
You have a cup of water and a large jug of water. What properties will remain the same between the two? What properties will be different between the two?
Mass: Same or different
Number of moles: Same or different
Number of particles: Same or different
Molar mass: Same or different

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:30
Jessica is traveling from miami, florida, to chicago, illinois. using the map, tell one way the land will change during the second half of her trip.
Answers: 1

Chemistry, 22.06.2019 07:30
Calculate the ratio of h+ ions to oh– ions at a ph = 7. find the concentration of h+ ions to oh– ions listed in table b of your student guide. then divide the h+ concentration by the oh– concentration. record this calculated ratio in table a of your student guide. compare your approximated and calculated ratios of h+ ions to oh– ions at a ph = 7. are they the same? why or why not? record your comparison in table a. what is the concentration of h+ ions at a ph = 7? mol/l what is the concentration of oh– ions at a ph = 7? mol/l what is the ratio of h+ ions to oh– ions at a ph = 7? : 1
Answers: 1

Chemistry, 22.06.2019 15:00
Answer explain why it is not possible to deduce a complete order of reactivity.
Answers: 3

Chemistry, 22.06.2019 22:30
Which process describes vaporization that takes place below the surface of a liquid? condensation melting boiling evaporation
Answers: 1
You know the right answer?
You have a cup of water and a large jug of water. What properties will remain the same between the t...
Questions


Mathematics, 20.12.2019 05:31

Mathematics, 20.12.2019 05:31




Biology, 20.12.2019 05:31


English, 20.12.2019 05:31



Chemistry, 20.12.2019 05:31


Mathematics, 20.12.2019 05:31

History, 20.12.2019 05:31

Mathematics, 20.12.2019 05:31

Mathematics, 20.12.2019 05:31



English, 20.12.2019 05:31



