Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 11:40
Which type of precipitation would most likely form when the surface air temperature is slightly below freezing and the air temperature increases as you move upward away from the ground?
Answers: 2


Chemistry, 23.06.2019 01:30
Polar bears give birth and hunt on sea ice. which of the following would polar bears survive during the melting of arctic ice? growing another layer of fur during summer migrate inland to search for different food sources staying put until the ice refreezes sticking to the usual diet of seals
Answers: 1

Chemistry, 23.06.2019 10:00
The temperature of a lead fishing weight rises from 26 °c to 38 °c as it absorbs 11.3 j of heat. what is the mass of the fishing weight in grams?
Answers: 1
You know the right answer?
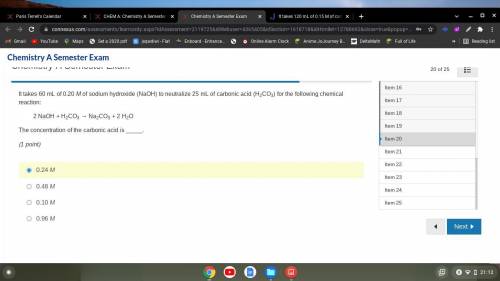
It takes 60 mL of 0.20 M of sodium hydroxide (NaOH) to neutralize 25 mL of carbonic acid (H2CO3) for...
Questions



Mathematics, 06.07.2019 18:20



Mathematics, 06.07.2019 18:20


Chemistry, 06.07.2019 18:20

Mathematics, 06.07.2019 18:20


Biology, 06.07.2019 18:20

Mathematics, 06.07.2019 18:20


Mathematics, 06.07.2019 18:20

Mathematics, 06.07.2019 18:20




Mathematics, 06.07.2019 18:20