
Chemistry, 13.12.2021 07:10 heybrothwrlogan
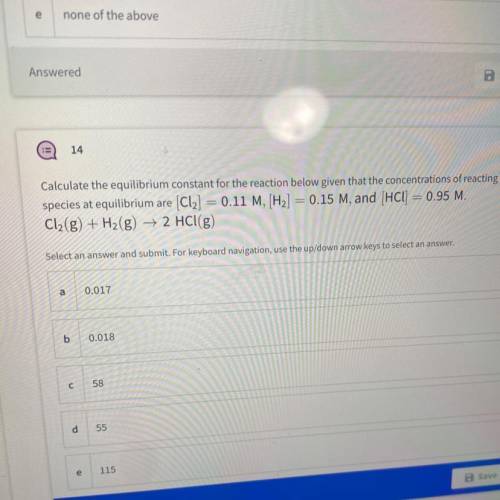
Calculate the equilibrium constant for the reaction below given that the concentrations of reacting
species at equilibrium are (Cl2] = 0.11 M, [H2] = 0.15 M, and (HCl) = 0.95 M.
Cl2(g) + H2(g) → 2 HCl(g)

Answers: 1


Another question on Chemistry


Chemistry, 23.06.2019 05:30
Stoichiometry- i need with 14 and 15! an explanation would be appreciated!
Answers: 1

Chemistry, 23.06.2019 05:30
Find the midpoint of a segment with endpoints of 4-3i and -2+7i
Answers: 2

Chemistry, 23.06.2019 11:20
Sandy is building a small toy car. he wants to use a balloon to power the toy car. he fills a balloon with air and then attaches a straw to the balloon. he tapes the balloon-straw combination to the car and then releases the end of the balloon. the toy moves forward as the air from the balloon comes out the back of the straw. what can sandy do to make the toy car move faster? a) use less air in the balloon. b) blow up the balloon more. c) use a longer straw. d) use larger tires.
Answers: 2
You know the right answer?
Calculate the equilibrium constant for the reaction below given that the concentrations of reacting...
Questions


Social Studies, 05.09.2019 03:10







History, 05.09.2019 03:10



Mathematics, 05.09.2019 03:10


Mathematics, 05.09.2019 03:10


Computers and Technology, 05.09.2019 03:10

Mathematics, 05.09.2019 03:10

English, 05.09.2019 03:10


History, 05.09.2019 03:10



