
Chemistry, 13.12.2021 07:10 daskatingpanda
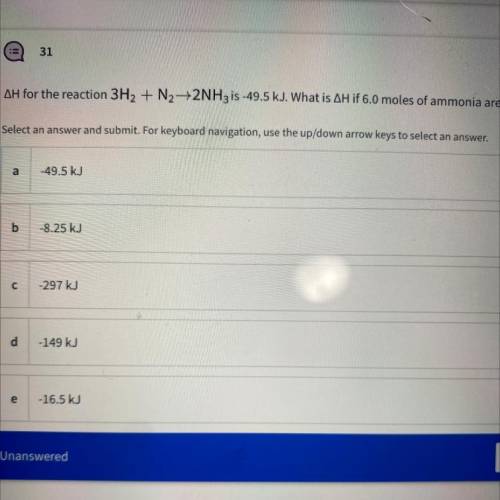
AH for the reaction 3H2 + N2 +2NH3 is -49.5 kJ. What is AH if 6.0 moles of ammonia are produced? Tor

Answers: 2


Another question on Chemistry


Chemistry, 23.06.2019 00:10
Find the missing probability in the table below a.0.10 b.40 c.0.80 d. 0.20
Answers: 2

Chemistry, 23.06.2019 11:30
How do you calculate the mass of a product when the amounts of more than one reactant are given?
Answers: 3

Chemistry, 23.06.2019 15:00
What is the volume in liters of 7500 g of helium atoms. assume stp conditions.
Answers: 1
You know the right answer?
AH for the reaction 3H2 + N2 +2NH3 is -49.5 kJ. What is AH if 6.0 moles of ammonia are produced?
T...
Questions

Mathematics, 07.04.2021 21:30




Mathematics, 07.04.2021 21:30

Computers and Technology, 07.04.2021 21:30


Mathematics, 07.04.2021 21:30

Mathematics, 07.04.2021 21:30



Computers and Technology, 07.04.2021 21:30

History, 07.04.2021 21:30

Mathematics, 07.04.2021 21:30

World Languages, 07.04.2021 21:30

Spanish, 07.04.2021 21:30

Mathematics, 07.04.2021 21:30

Biology, 07.04.2021 21:30


Spanish, 07.04.2021 21:30



