
Chemistry, 10.12.2021 02:00 shadestephen25
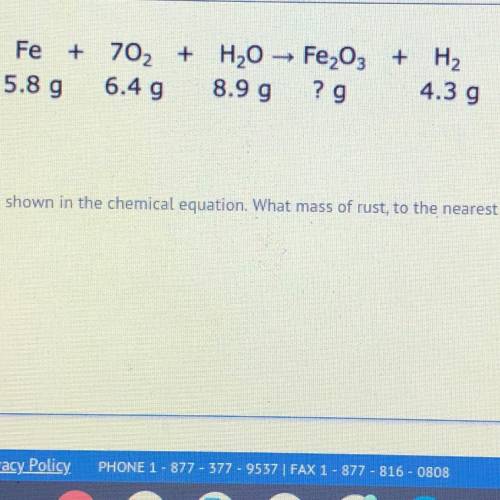
iron reacts with oxygen and water to create rust and hydrogen gas as shown in the chemical equation. what mass of rust, to the nearest hundredth gram, is produced in this reaction?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 16:30
Why should the scientific method be used to answer a question? a. it provides a way to test an idea without any bias. b. it provides a way to test a hypothesis. c. it provides a way to ensure all hypotheses are proven correct. d. it provides a way to quickly turn a hypothesis into a scientific theory.
Answers: 1

Chemistry, 21.06.2019 23:00
The agent of mechanical weathering in which rock is worn away by the grinding action of other rock particles is call
Answers: 1

Chemistry, 22.06.2019 01:50
7. what temperature is need to just dissolve 50 g of nh4cl in 75 g of water? '
Answers: 1

Chemistry, 22.06.2019 03:00
Zoe is investigating the composition of substance a, an unknown substance. using chemical processes, she analyzes substance a and determines it is composed of sodium, oxygen, and hydrogen atoms in a ratio of 1 : 1 : 1. what is substance a? a. a compound b. an element c. a heterogeneous mixture d. a homogeneous mixture
Answers: 1
You know the right answer?
iron reacts with oxygen and water to create rust and hydrogen gas as shown in the chemical equation....
Questions

Mathematics, 02.08.2019 15:00

Social Studies, 02.08.2019 15:00

Mathematics, 02.08.2019 15:00

History, 02.08.2019 15:00

Chemistry, 02.08.2019 15:00


Arts, 02.08.2019 15:00

Biology, 02.08.2019 15:00

Social Studies, 02.08.2019 15:00




Chemistry, 02.08.2019 15:00



Mathematics, 02.08.2019 15:00


English, 02.08.2019 15:00

Arts, 02.08.2019 15:00



