
Chemistry, 09.12.2021 06:20 QueenNerdy889
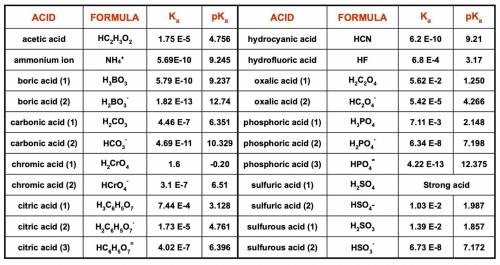
1. List the species present at equilibrium in a solution with the following
composition:
NH4Cl = 0.0200 mol/L NaOH = 0.0430 mol/L
H2SO4 = 0.0150 mol/L NaNO3 = 0.0100 mol/L
2. Write the n equations for n unknowns describing the equilibrium composition of
this system.
3. Make a spreadsheet and use Excel’s Solver function to determine the equilibrium
pH and concentrations of all species in this solution.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 02:30
When svante arrhenius first proposed his acid-base theory, he was a doctoral candidate. his professors thought his ideas were unfounded. within a decade, the arrhenius theory of acid-base was widely accepted and praised within the scientific world. arrhenius defined acids as compounds having ionizable hydrogen and bases as compounds with ionizable a) barium. b) hydronium. c) hydroxide. d) oxygen.
Answers: 3

Chemistry, 22.06.2019 11:10
Which of the following shapes would represent a molecule with two bonded atoms and 3 lone pairs on only one of them , trigonal planar , bent , trigonal pyramidal , linear
Answers: 1

Chemistry, 22.06.2019 12:00
Marcel just purchased 1.69 grams of iron fillings in order to make living putty for his 6 year old niece. how many moles of iron are made in his sample?
Answers: 1

Chemistry, 22.06.2019 13:50
Abeaker with 2.00×102 ml of an acetic acid buffer with a ph of 5.000 is sitting on a benchtop. the total molarity of acid and conjugate base in this buffer is 0.100 m. a student adds 4.70 ml of a 0.360 m hcl solution to the beaker. how much will the ph change? the pka of acetic acid is 4.740.
Answers: 1
You know the right answer?
1. List the species present at equilibrium in a solution with the following
composition:
Questions

Computers and Technology, 24.10.2019 18:43


Computers and Technology, 24.10.2019 18:43

English, 24.10.2019 18:43



English, 24.10.2019 18:43

Computers and Technology, 24.10.2019 18:43


Computers and Technology, 24.10.2019 18:43



Mathematics, 24.10.2019 18:43





Computers and Technology, 24.10.2019 18:43


English, 24.10.2019 18:43



