
Chemistry, 09.12.2021 05:50 natnerd4671
For parts of the free-response question that require calculations, clearly show the method used and the steps involved in arriving at your answers. You must show your work to receive credit for your answer. Examples and equations may be included in your answers where appropriate.
2NO2(g)+F2(g)→NO2F(g)
ΔH∘rxn=−284kJ/molrxn
NO2(g) and F2(g) can react to produce NO2F(g), as represented above. A proposed mechanism for the reaction has two elementary steps, as shown below.
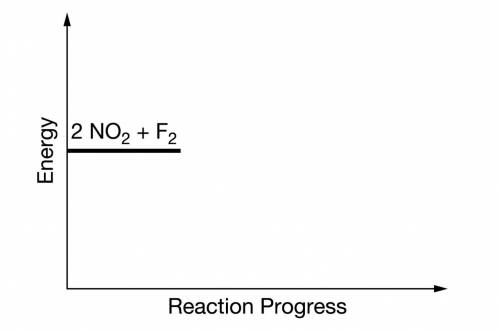
(b) On the incomplete reaction energy diagram below, draw a curve that shows the following two details.
The relative activation energies of the two elementary steps
The enthalpy change of the overall reaction

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 17:50
Which best describes why nh4+ can form an ionic bond with cl-?
Answers: 3

Chemistry, 22.06.2019 16:40
Let the ed50 of a recreational drug be defined as the amount required for 50% of a test group to feel high or get a buzz. if the ed50 value of ethanol is 470 mg/kg body mass, what dose would a 70 kg party goer need to quickly consume in order to have a 50% chance of getting a buzz? 235 mg 470 mg 32,900 mg 35,000,000 mg
Answers: 3


Chemistry, 23.06.2019 16:00
How many moles of sulfuric acid (h2so4) are needed to react completely with 6.8 moles of lithium hydroxide (lioh)? 2lioh + h2so4 → li2so4 + 2h2o a) 3.4 mol h2so4 b) 6.8 mol h2so4 c) 10.2 mol h2so4 d) 13.6 mol h2so4 a) 3.4 mol h2so4
Answers: 1
You know the right answer?
For parts of the free-response question that require calculations, clearly show the method used and...
Questions


Mathematics, 17.12.2020 08:10


Mathematics, 17.12.2020 08:10


Mathematics, 17.12.2020 08:10

Mathematics, 17.12.2020 08:10

Mathematics, 17.12.2020 08:10

Chemistry, 17.12.2020 08:10

Mathematics, 17.12.2020 08:10


Geography, 17.12.2020 08:10

Mathematics, 17.12.2020 08:10


Chemistry, 17.12.2020 08:10

Mathematics, 17.12.2020 08:10

Chemistry, 17.12.2020 08:10

Mathematics, 17.12.2020 08:10

Mathematics, 17.12.2020 08:10

Mathematics, 17.12.2020 08:10



