
A substance (A) reacts to form another substance (B):
3A(g)
↔
2B(g)
The reaction is run at a particular temperature with the concentrations of A and B monitored over time and plotted in the graph. At what time was equilibrium first reached and what is the approximate value of the equilibrium constant?
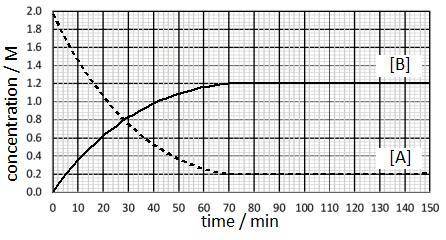

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 23:00
When determining the shape of a molecule, it is important to draw a lewis dot structure first in order to see the total number a. electrons within the moleculeb. bonding and unshared pairs around central atomc. unshared pair within the molecule( i really need it )
Answers: 1

Chemistry, 22.06.2019 09:40
Consider this initial-rate data at a certain temperature for the reaction described by
Answers: 1

Chemistry, 22.06.2019 20:00
Nitrogen dioxide decomposes according to the reaction 2 no2(g) ⇌ 2 no(g) + o2(g) where kp = 4.48 × 10−13 at a certain temperature. if 0.70 atm of no2 is added to a container and allowed to come to equilibrium, what are the equilibrium partial pressures of no(g) and o2(g)
Answers: 2

Chemistry, 22.06.2019 21:30
How can the periodic table be used to predict the behavior of elements?
Answers: 1
You know the right answer?
A substance (A) reacts to form another substance (B):
3A(g)
↔
2B(g)
The r...
↔
2B(g)
The r...
Questions


Mathematics, 19.10.2019 10:10

Mathematics, 19.10.2019 10:10


Biology, 19.10.2019 10:10





Mathematics, 19.10.2019 10:10




Mathematics, 19.10.2019 10:20


Mathematics, 19.10.2019 10:20

Physics, 19.10.2019 10:20

Mathematics, 19.10.2019 10:20


Physics, 19.10.2019 10:20



