A company claims that one of their antacid
tablets contains 0.400g of calcium. A student did
...

Chemistry, 08.12.2021 22:00 tyliyahmiles99
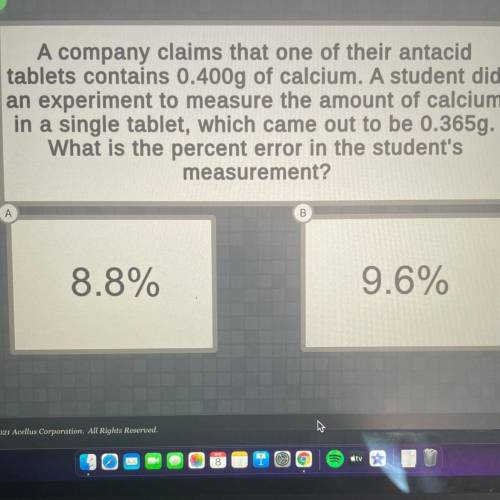
A company claims that one of their antacid
tablets contains 0.400g of calcium. A student did
an experiment to measure the amount of calcium
in a single tablet, which came out to be 0.365g.
What is the percent error in the student's
measurement?
А
B
8.8%
9.6%

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 13:50
Read the chemical equation. 2c2h2 + 5o2 → 4co2 + 2h2o which of the following statements would be correct if one mole of c2h2 was used in this reaction? one mole of oxygen was used in this reaction. five moles of oxygen were used in this reaction. four moles of carbon dioxide were produced from this reaction. two moles of carbon dioxide were produced from this reaction.
Answers: 3

Chemistry, 22.06.2019 17:00
The msds for glacial acetic acid says that it is a flammable liquid that can severely burn any human tissue it comes in contact with. it reacts with bases, various metals, and strong oxidizing agents. its vapors can form explosive mixtures with air.
Answers: 1

Chemistry, 22.06.2019 18:00
How is energy related to the change of state represented by the model? atoms gain energy as a solid changes to a liquid. atoms gain energy as a solid changes to a gas. atoms lose energy as a solid changes to a liquid. atoms lose energy as a solid changes to a gas.
Answers: 3
You know the right answer?
Questions

Computers and Technology, 10.03.2020 09:12
















Mathematics, 10.03.2020 09:12





