D
Tare the balance. Put calorimeter (no lid)
on the balance. Measure the mass to the
n...

Chemistry, 08.12.2021 01:00 mexicanvanilla
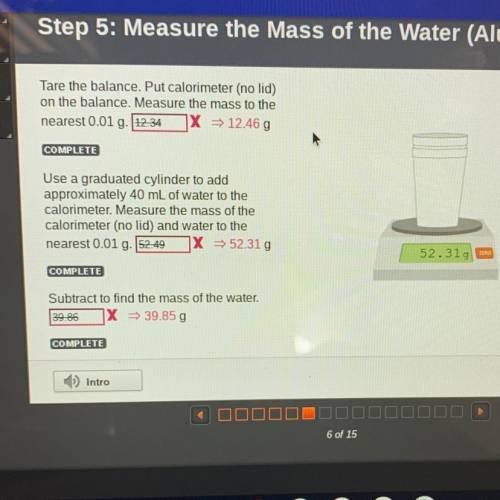
D
Tare the balance. Put calorimeter (no lid)
on the balance. Measure the mass to the
nearest 0.01 g. 12.34 X = 12.46 g
COMPLETE
Use a graduated cylinder to add
approximately 40 mL of water to the
calorimeter. Measure the mass of the
calorimeter (no lid) and water to the
nearest 0.01 g. 52.49
X = 52.31g
52.319 EEN
COMPLETE
Subtract to find the mass of the water.
39.86
X = 39.85 g
COMPLETE

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 06:30
(1.6 × 10-19)(5.0 × 106) = c × 10d identify the missing numbers below to show the result of multiplying the numbers.
Answers: 1

Chemistry, 22.06.2019 09:00
The nuclear fission process releases neutrons and question 27 options: alpha particles electrons energy beta particles
Answers: 1

Chemistry, 22.06.2019 09:30
Right anwser gets marked brainliest newton's discovery concerning how fast an object will change speed is the: 1st law 2nd law 3rd law universal gravitation
Answers: 1

Chemistry, 22.06.2019 14:30
The three types is stress that act on earths rocks are compression, tension, and
Answers: 1
You know the right answer?
Questions

Biology, 14.06.2021 18:10

Biology, 14.06.2021 18:10




Mathematics, 14.06.2021 18:10


Mathematics, 14.06.2021 18:10

Mathematics, 14.06.2021 18:10








Mathematics, 14.06.2021 18:10


Mathematics, 14.06.2021 18:10

Mathematics, 14.06.2021 18:10



