
Chemistry, 06.12.2021 22:10 kyliegriffis
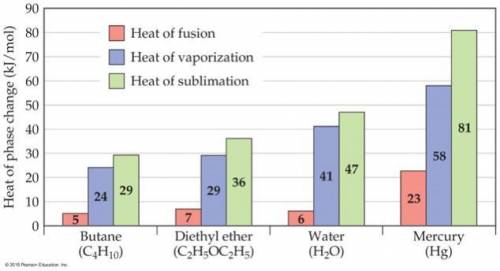
Consider the figure below. In the case of butane, diethyl ether, and water, the heat of vaporization is considerably larger than heat of fusion. Explain this phenomenon. Note that the nature of intermolecular interactions does not change through the phases because the molecules does not undergo chemical changes.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 02:00
Write a hypothesis that answers the lesson question, “while observing a chemical reaction, how can you tell which reactant is limiting? ” hypothesis: if a substance is the limiting reactant, then . . because . .
Answers: 1

Chemistry, 22.06.2019 09:20
What happened to the amount of carbon dioxide in the atmosphere from 2010–2017?
Answers: 1

Chemistry, 22.06.2019 10:50
Determine the empirical formula for succinic acid that is composed of 40.60% carbon, 5.18% hydrogen, and 54.22% oxygen.
Answers: 1

Chemistry, 22.06.2019 12:30
Nebulae are enormous clouds in outer space. they are made mostly of hydrogen gas, helium gas, and dust. some nebulae glow brightly, while others do not. the stars that people see are huge, bright balls of glowing gas. they are made mostly of hydrogen and helium. which statement correctly describes other ways in which nebulae and stars are different? a. stars can form inside a nebula but a nebula can never be produced by any star. b. a star always has a higher density than a nebula. c. stars can never form inside a nebula but a nebula can be produced by any star. d. a nebula always has a higher density than a star.
Answers: 3
You know the right answer?
Consider the figure below. In the case of butane, diethyl ether, and water, the heat of vaporization...
Questions

Mathematics, 28.09.2019 18:20



Chemistry, 28.09.2019 18:20


Physics, 28.09.2019 18:20




Spanish, 28.09.2019 18:20


Mathematics, 28.09.2019 18:30


English, 28.09.2019 18:30

History, 28.09.2019 18:30

Social Studies, 28.09.2019 18:30

Mathematics, 28.09.2019 18:30


Mathematics, 28.09.2019 18:30

English, 28.09.2019 18:30



