
Chemistry, 05.12.2021 22:40 azireyathurmond1
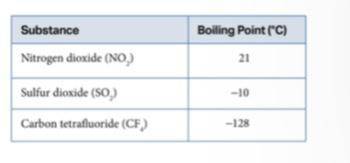
Sulfur dioxide (SO2) and nitrogen dioxide (NO2) both have dipoles, and carbon tetrafluoride (CF4) is nonpolar. All the molecules have relatively similar masses. What could account for the difference in their boiling points? GIVING BRAINLIEST OUT.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 23:40
If the atomic mass of an atom is 34 and the atom contains 13 protons, how many neutrons does the atom contain?
Answers: 2

Chemistry, 22.06.2019 09:20
What will most likely happen when two bromine atoms bond together?
Answers: 3

Chemistry, 22.06.2019 13:00
Asubstance is a good conductor of electricity which of the following best explains a probable position of the substance in a periodic table
Answers: 3

Chemistry, 22.06.2019 17:00
Astable electron arrangement for an atom is one that does not easily change. how is this arrangement arrived at? a. valence electrons are transferred or shared to create a full outer shell of electrons. b. valence electrons are discarded into space to create a full outer shell of electrons. c. protons (positive charge) pair with valence electrons (negative charge) to create a strong bond. d. outer shells with valence electrons are transferred or shared.
Answers: 2
You know the right answer?
Sulfur dioxide (SO2) and nitrogen dioxide (NO2) both have dipoles, and carbon tetrafluoride (CF4) is...
Questions


Mathematics, 23.08.2021 19:40

Mathematics, 23.08.2021 19:40



History, 23.08.2021 19:40



Biology, 23.08.2021 19:40


Mathematics, 23.08.2021 19:40

Mathematics, 23.08.2021 19:40




English, 23.08.2021 19:40






