Points: 99

Chemistry, 05.12.2021 20:30 SorayaC9669
Hello! Everyone:) I was just wondering if you can help me with my test.
Points: 99
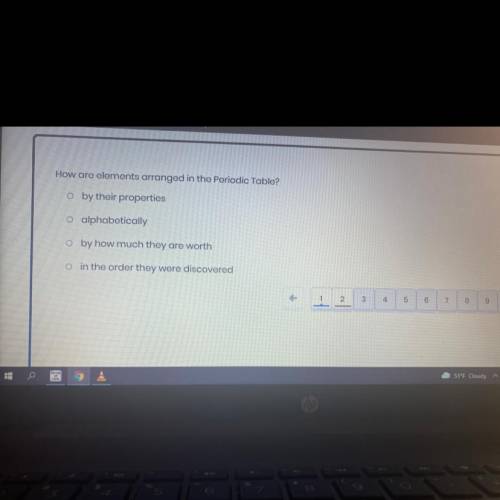

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 14:00
Which of the following statements is true? question 4 options: nuclear decay rates vary with the conditions of the reaction, but chemical reaction rates do not. chemical reaction rates vary with the conditions of the reaction, but nuclear decay rates do not. neither chemical reaction rates nor nuclear decay rates vary with the conditions of the reaction. both chemical reaction rates and nuclear decay rates vary with the conditions of the reaction.
Answers: 1

Chemistry, 23.06.2019 06:00
When hydrogen peroxide (h2o2) is added to potassium iodide (ki) solution, the hydrogen peroxide decomposes into water (h2o) and oxygen (o2). the chemical equation for the decomposition reaction is: 2h2o2—> 2h2o + o2. what is the role of the potassium iodide in this reaction? a. reactant. b. product. c. precipitate. d. catalyst.
Answers: 1

Chemistry, 23.06.2019 06:30
The molar mass of cu is 63.55 g/mol. the number of grams of cu produced in this reaction is
Answers: 3

Chemistry, 23.06.2019 10:30
Ireally need ! calcium metal reacts with a potassium chloride solution to form calcium chloride and potassium ions. balance this reaction. (s) + (aq) → cacl2(s) + +(aq) a) 1, 2, 1, 2 b) 1, 2, 1, 1 c) 1, 1, 1, 1 d) 2, 1, 2, 1
Answers: 1
You know the right answer?
Hello! Everyone:) I was just wondering if you can help me with my test.
Points: 99
Points: 99
Questions

English, 04.12.2020 22:40


Chemistry, 04.12.2020 22:40



English, 04.12.2020 22:40

History, 04.12.2020 22:40





Mathematics, 04.12.2020 22:40




Mathematics, 04.12.2020 22:40


Mathematics, 04.12.2020 22:40

Mathematics, 04.12.2020 22:40

History, 04.12.2020 22:40



