
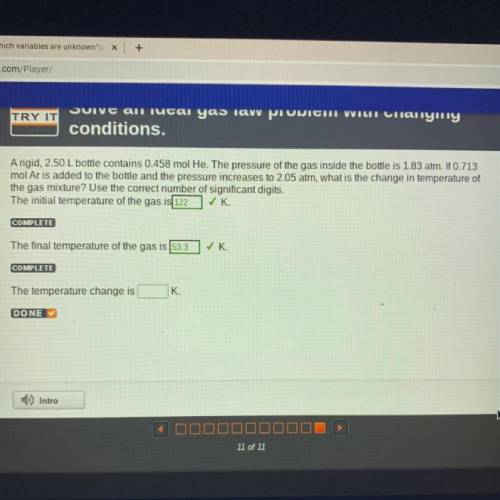
A rigid, 2.50 L bottle contains 0.458 mol He. The pressure of the gas inside the bottle is 1.83 atm. If 0.713
mol Ar is added to the bottle and the pressure increases to 2.05 atm, what is the change in temperature of
the gas mixture? Use the correct number of significant digits.
The initial temperature of the gas is 122 ✓K
COMPLETE
The final temperature of the gas is 53.3
✓ K.
COMPLETE
The temperature change is
K.
DONE

Answers: 1


Another question on Chemistry

Chemistry, 23.06.2019 12:30
If you reacted 450 g of trimethylgallium with 300 g of arsine, what mass of gaas could you make?
Answers: 1

Chemistry, 23.06.2019 16:00
The electron configuration for chromium is 1s22s22p63s23p63d54s1 instead of 1s22s22p63s23p63d44s1. the configuration is an exception to the pauli exclusion principle heisenberg uncertainty principle aufbau principle schrödinger equation
Answers: 3

Chemistry, 23.06.2019 21:30
Which particles make up the nucleus of an atom? a. protons and electrons b. neutrons and electrons c. protons only d. protons and neutrons e. neutrons only
Answers: 2

Chemistry, 23.06.2019 22:00
The following compounds, listed with their boiling points, are liquid at –10ºc: butane, –0.5ºc; ethanol, 78.3ºc; toluene, 110.6ºc. at –10ºc, which of these liquids would you expect to have the highest vapor pressure? which the lowest? explain. (4 points)
Answers: 1
You know the right answer?
A rigid, 2.50 L bottle contains 0.458 mol He. The pressure of the gas inside the bottle is 1.83 atm....
Questions

Physics, 01.04.2021 02:00


History, 01.04.2021 02:00

Mathematics, 01.04.2021 02:00

Mathematics, 01.04.2021 02:00

Mathematics, 01.04.2021 02:00

English, 01.04.2021 02:00

Social Studies, 01.04.2021 02:00



Mathematics, 01.04.2021 02:00


Mathematics, 01.04.2021 02:00

Mathematics, 01.04.2021 02:00

English, 01.04.2021 02:00

History, 01.04.2021 02:00

Chemistry, 01.04.2021 02:00

Mathematics, 01.04.2021 02:00

Mathematics, 01.04.2021 02:00

Computers and Technology, 01.04.2021 02:00



