
Chemistry, 02.12.2021 21:30 Hollywood0122
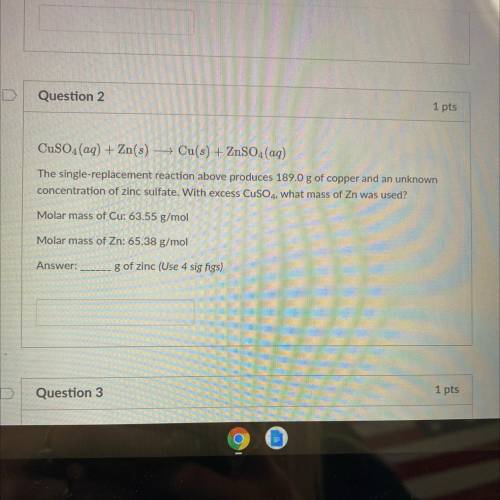
CuSO4(aq) + Zn(s) + Cu(s) + ZnSO4(aq)
The single-replacement reaction above produces 189.0 g of copper and an unknown
concentration of zinc sulfate. With excess CuSO4, what mass of Zn was used?
Molar mass of Cu: 63.55 g/mol
Molar mass of Zn: 65.38 g/mol
g of zinc (Use 4 sig figs)

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 17:00
What were the success and failures that came to boyle’s excitements?
Answers: 1

Chemistry, 21.06.2019 20:10
What can be added to the examples section of each circle? endothermic: ice melting into water, and a heat pack becoming warm exothermic: a glow stick glowing, and fireworks exploding endothermic: ice melting into water, and an instant ice pack turning cold exothermic: fireworks exploding, and gasoline burning endothermic: a glow stick glowing, and a heat pack becoming warm exothermic: an instant ice pack turning cold, and ice melting into water endothermic: gasoline burning, and an instant ice pack turning cold exothermic: ice melting into water, and an instant ice pack turning cold
Answers: 1

Chemistry, 22.06.2019 08:00
This classification of drug typically changes the brain's chemistry and reduces its ability to create its own endorphins.
Answers: 1

Chemistry, 22.06.2019 13:00
12. calculate the hydroxide ion concentration of a solution with ph = 3.25. show all calculations leading to an answer
Answers: 3
You know the right answer?
CuSO4(aq) + Zn(s) + Cu(s) + ZnSO4(aq)
The single-replacement reaction above produces 189.0 g of co...
Questions



Mathematics, 04.02.2021 21:30

Mathematics, 04.02.2021 21:30

Mathematics, 04.02.2021 21:30



Mathematics, 04.02.2021 21:30



Spanish, 04.02.2021 21:30



Spanish, 04.02.2021 21:30


Mathematics, 04.02.2021 21:30


Mathematics, 04.02.2021 21:30

Mathematics, 04.02.2021 21:30



