9.
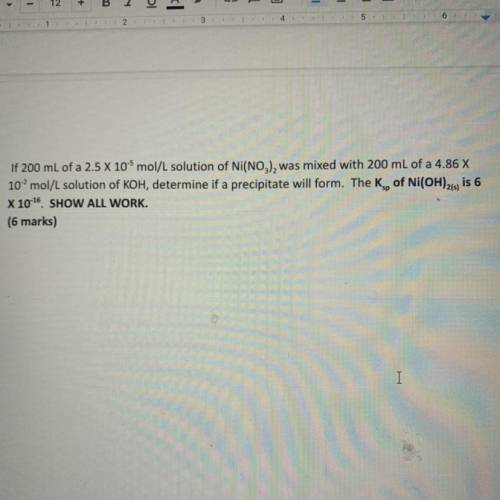
If 200 mL of a 2.5 x 10-5 mol/L solution of Ni(NO3), was mixed with 200 mL of a 4.86 X
10...

Chemistry, 02.12.2021 18:10 kstyleszdance
9.
If 200 mL of a 2.5 x 10-5 mol/L solution of Ni(NO3), was mixed with 200 mL of a 4.86 X
102 mol/L solution of KOH, determine if a precipitate will form. The Kg, of Ni(OH)21, is 6
X 10-16 SHOW ALL WORK.
(6 marks)

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Which of these properties, used alone, would be least useful in identifying most minerals? a. color b. luster c. streak d. density
Answers: 2

Chemistry, 22.06.2019 10:30
What determines the average kinetic energy of the particles in a gas? a. the number of collisions b. the number of particles c. the size of the particles d. the temperature
Answers: 1

Chemistry, 22.06.2019 12:00
Which of the following is an example of physical change not a chemical change? a) a log gives off heat and light as it burns. b) a tree stores energy from the sun in its fruit. c) a penny lost in the grass slowly changes color. d) a water pipe freezes and cracks on a cold night.
Answers: 2

Chemistry, 22.06.2019 17:00
Which statement is true about a catalyst? a: a catalyst decreases the rate of the reaction. b. a catalyst is consumed during a chemical reaction. c. a catalyst lowers the activation energy of a reaction. d. a catalyst increases the reactant concentration during a reaction.
Answers: 1
You know the right answer?
Questions


Mathematics, 06.01.2021 17:30


Mathematics, 06.01.2021 17:30

Social Studies, 06.01.2021 17:30

English, 06.01.2021 17:30


Mathematics, 06.01.2021 17:30


SAT, 06.01.2021 17:30




Chemistry, 06.01.2021 17:30


History, 06.01.2021 17:30

Social Studies, 06.01.2021 17:30

Mathematics, 06.01.2021 17:30




