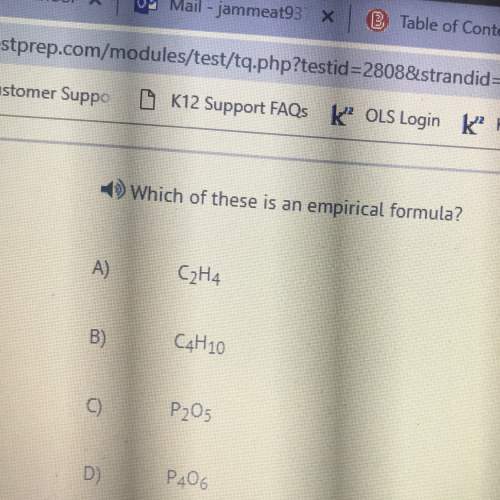
Chemistry, 23.11.2021 03:10 guazet7650
What is the pH of a buffer that results when 0.40 mol NaHCO2 is mixed with 100.0 mL of 2.00 M HCl(aq) and diluted with water to 250 mL? (Ka of HCO2H = 1.8 × 10–4)

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 01:30
Arollercoaster car at the top of a hill has potential energy kinetic energy chemical energy light energy
Answers: 1

Chemistry, 22.06.2019 06:30
Design techniques and materials that reduce the negative environmental impact of a structure are referred to as
Answers: 2

Chemistry, 22.06.2019 11:30
Voltaic cells produce a positive overall charge. what does this indicate? a. the reaction is likely to be endothermic. b. the reaction is spontaneous. c. the reaction is not likely to occur. d. the reaction is not spontaneous.
Answers: 3

Chemistry, 22.06.2019 11:30
For each of the following compounds, decide whether the compound's solubility in aqueous solution changes with ph. if the solubility does change, pick the ph at which you'd expect the highest solubility. you'll find ksp data in the aleks data tab. compounds does solubility change with ph
Answers: 3
You know the right answer?
What is the pH of a buffer that results when 0.40 mol NaHCO2 is mixed with 100.0 mL of 2.00 M HCl(aq...
Questions



English, 18.04.2020 01:42

Mathematics, 18.04.2020 01:42


Mathematics, 18.04.2020 01:42


Chemistry, 18.04.2020 01:42

Mathematics, 18.04.2020 01:42




History, 18.04.2020 01:42

Mathematics, 18.04.2020 01:42



English, 18.04.2020 01:42


Computers and Technology, 18.04.2020 01:42