
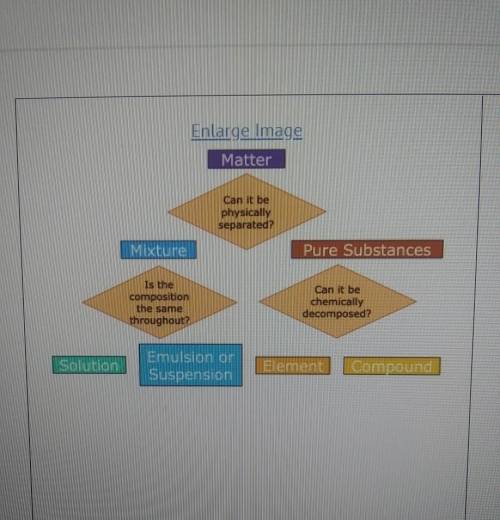
A student has a sample of ocean water that they take to science class. what steps could be taken to determine to classify the ocean water as a suspension, solution, element, or compound?
A) Examine the pH of the sample. If the pH is exactly neutral, it is a pure substance that is a compound.
B) Evaporate the water. If salt is left behind, the sample is a mixture that can be defined as a solution.
C) Let the sample settle. If the salt settles to the bottom, it is a mixture that is classified as a solution.
D) Observe the diffraction of light as it moved through the sample. If light does not scatter, it is a pure substance that is elemental.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 20:30
This chart represents the melting point of several substance. what besy explains the high melting point of the salt?
Answers: 2

Chemistry, 22.06.2019 09:10
When a nucleus absorbs a neutron and then breaks apart, there are many products of the reaction. what is not a product of a nuclear fission reaction
Answers: 1

Chemistry, 22.06.2019 17:40
Areaction in which products can react to re-form reactants is
Answers: 1

Chemistry, 23.06.2019 01:30
Ariver current has a velocity of 5km/h relative to the shore, and a boat moves in the same direction as the current at 5 km/h relative to the river. how can the velocity of the boat relative to the shore be calculated?
Answers: 1
You know the right answer?
A student has a sample of ocean water that they take to science class. what steps could be taken to...
Questions









English, 02.08.2019 23:40

English, 02.08.2019 23:40




Social Studies, 02.08.2019 23:40

Mathematics, 02.08.2019 23:40


History, 02.08.2019 23:40

Chemistry, 02.08.2019 23:40





