
Chemistry, 10.11.2021 20:10 theatergeek005
Topics: Fundamentals of electrochemistry; Electrochemical cells
Use general chemistry rules for significant figures.
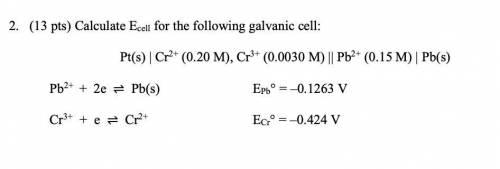
2. (13 pts) Calculate Ecell for the following galvanic cell:
Pt(s) | Cr2+ (0.20 M), Cr3+ (0.0030 M) || Pb2+ (0.15 M) | Pb(s)
Pb2+ + 2e ⇌ Pb(s) EPb° = –0.1263 V
Cr3+ + e ⇌ Cr2+ ECr° = –0.424 V

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:00
Which produce would best increase the amount of heat energy that is actually gained by calorimeter b
Answers: 1

Chemistry, 22.06.2019 01:00
Which type of orbits are found in the principal energy level n = 2 a - s b - s, f c - s, d d - s, p e - s, p, d
Answers: 1


Chemistry, 22.06.2019 09:00
If you chip a tooth, most likely you will go to the dentist to have the missing material filled in. currently the material used to fill in teeth is a polymer that is flexible when put in, yet is hardened to the strength of a tooth after irradiation with blue light at a wavelength of 461 nm. what is the energy in joules for a photon of light at this wavelength?
Answers: 1
You know the right answer?
Topics: Fundamentals of electrochemistry; Electrochemical cells
Use general chemistry rules for si...
Questions


English, 07.05.2020 15:00








Mathematics, 07.05.2020 15:00






Health, 07.05.2020 15:00

Mathematics, 07.05.2020 15:00





