
Determining Equilibrium
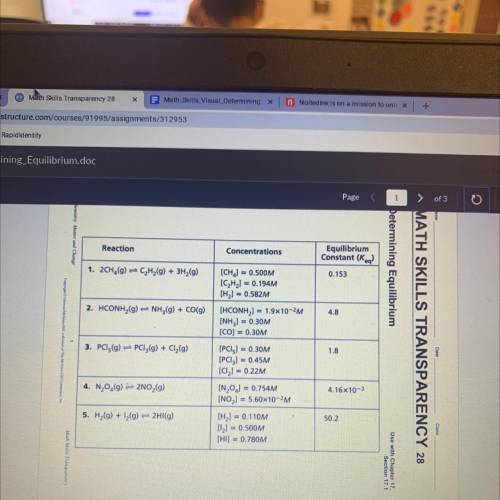
The equilibrium constants for the reactions in the table are correct at a certain
temperature. The concentrations given in the table, however, may or may not be correct
when the system is at equilibrium at that temperature. Use the information in the table to
answer the following questions.
1.On the basis of the K, values given in the table, which reaction mixture contains the largest
amount of product(s) when at equilibrium?
2. Which reaction mixture contains the largest amount of reactants when at
equilibrium? Explain.
3. Which reactions in the table have concentrations that represent the systems at
equilibrium? Explain.
4. For each reaction that is not at equilibrium, change the concentration of only one
of the reactants or products so that the ratio represents the system at equilibrium.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 05:30
The climate of the continental united states is generally 1. tropical 2. temperate 3. arctic 4. highland
Answers: 1


Chemistry, 22.06.2019 06:00
How much would the freezing point of water decrease if 4 mol of sugar were added to 1 kg of water(k=1.86 c/mol/kg for water and i=1 for sugar
Answers: 1

Chemistry, 22.06.2019 08:30
Which of the following would be an accurate picture of the earth during the summer time of the northern hemisphere?
Answers: 1
You know the right answer?
Determining Equilibrium
The equilibrium constants for the reactions in the table are correct at a...
Questions


Health, 11.10.2020 14:01

Mathematics, 11.10.2020 14:01



Social Studies, 11.10.2020 14:01

Mathematics, 11.10.2020 14:01

Mathematics, 11.10.2020 14:01


Spanish, 11.10.2020 14:01



Mathematics, 11.10.2020 14:01

Arts, 11.10.2020 14:01

Social Studies, 11.10.2020 14:01

Mathematics, 11.10.2020 14:01

French, 11.10.2020 14:01


Mathematics, 11.10.2020 14:01




