Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 04:00
What three natural resources are found in the great lakes region
Answers: 2

Chemistry, 22.06.2019 23:10
Match the formula for the following compound: magnesium sulfate heptahydratemgs·7h2omg2so4·7h2omg(so4)2·7h2omgso4·7h2o
Answers: 1

Chemistry, 23.06.2019 00:30
Balance the following reaction. as2s3 + 9o2 → 2as2o3 + so2
Answers: 2
You know the right answer?
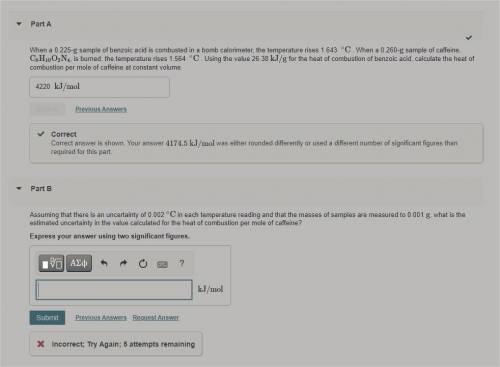
Assuming that there is an uncertainty of 0.002 ∘C in each temperature reading and that the masses of...
Questions

Health, 02.03.2021 21:40




Mathematics, 02.03.2021 21:40

Mathematics, 02.03.2021 21:40

Mathematics, 02.03.2021 21:40


Mathematics, 02.03.2021 21:40

Biology, 02.03.2021 21:40

History, 02.03.2021 21:40


Mathematics, 02.03.2021 21:40




Mathematics, 02.03.2021 21:40

Mathematics, 02.03.2021 21:40


Mathematics, 02.03.2021 21:40