Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 16:30
10-14. (a) when 100.0 ml of weak acid ha were titrated with 0.093 81 m naoh, 27.63 ml were required to reach the equivalence point. find the molarity of ha. (b) what is the formal concentration of a- at the equivalence point? (c) the ph at the equivalence point was 10.99. find pk. for ha. (d) what was the ph when only 19.47 ml of naoh had been added?
Answers: 1

Chemistry, 21.06.2019 22:30
Which of these sequences lists the correct order for the creation of sedimentary rock from sediment? a. deposition, burial, compaction, cementation b. burial, deposition, compaction, cementation c. compaction, deposition, burial, cementation d. cementation, deposition, burial, compaction
Answers: 1

Chemistry, 22.06.2019 04:20
Which formula can be used to calculate the molar mass of ammonia (nh3)? molar mass of n + molar mass of h 3 × molar mass of n + molar mass of h molar mass of n + 3 × molar mass of h 3 × molar mass of n + 3 × molar mass of h
Answers: 1

Chemistry, 22.06.2019 04:30
What are the primary responsibilities of a chemical engineer involved in "r& d"? develop large scale manufacturing operations discover new products and processes training of new chemists determine products needed by consumers
Answers: 2
You know the right answer?
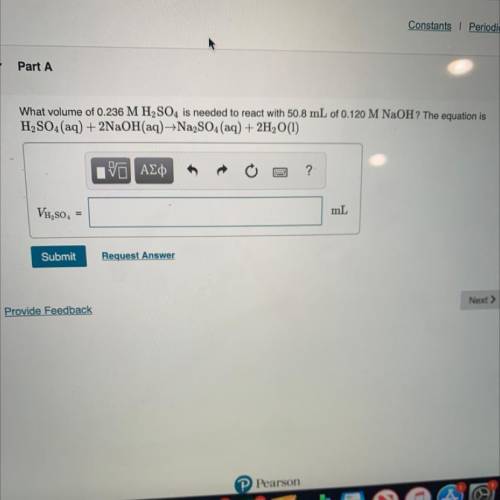
What volume of 0.236 M H2SO4 is needed to react with 50.8 mL of 0.120 M NaOH? The equation is
H2SO...
Questions



SAT, 15.01.2020 07:31

Chemistry, 15.01.2020 07:31

Mathematics, 15.01.2020 07:31

Mathematics, 15.01.2020 07:31

History, 15.01.2020 07:31




History, 15.01.2020 07:31



History, 15.01.2020 07:31

Chemistry, 15.01.2020 07:31


History, 15.01.2020 07:31


Mathematics, 15.01.2020 07:31