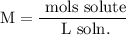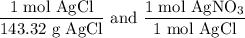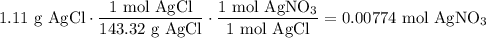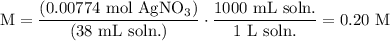Chemistry, 30.10.2021 19:00 jchavez0790
HELPPP PLS IM DESPRATE MAJOR POINTS NEED ASAP
We add excess NaCl solution (58.44 g/mol) to 38 mL of a solution of silver nitrate (AgNO3 169.88 g/mol), to form insoluble solid AgCl. When it has been dried and weighed, the mass of AgCl (143.32 g/mol) is found to be 1.11 grams.
What is the molarity of the original AgNO3 solution? The formula weight of NaNO3 is 85.00 g/mol.
Answer in units of M.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 08:00
What are the similarities of physical and chemical change ?
Answers: 1

Chemistry, 22.06.2019 15:30
The reactions of photosynthesis occur in the of plant cell? a.mitochondria. b. lysosomes. c. chloroplasts. d. chlorophyll
Answers: 1

Chemistry, 22.06.2019 15:50
How many moles of potassium hydroxide are needed to completely react with 2.94 moles of aluminum sulfate
Answers: 1

Chemistry, 22.06.2019 23:00
How does the value of the equilibrium constant show that a reaction reaches equilibrium very quickly? (a) the equilibrium constant is large. (b) the equilibrium constant is small. (c) the equilibrium constant is zero. (d) the value of the equilibrium constant does not show how quickly a reaction comes to equilibrium.
Answers: 1
You know the right answer?
HELPPP PLS IM DESPRATE MAJOR POINTS NEED ASAP
We add excess NaCl solution (58.44 g/mol) to 38 mL o...
Questions


History, 20.04.2021 09:50

Mathematics, 20.04.2021 09:50

Mathematics, 20.04.2021 09:50

Chemistry, 20.04.2021 09:50




Biology, 20.04.2021 09:50

Physics, 20.04.2021 09:50

Mathematics, 20.04.2021 14:00

Computers and Technology, 20.04.2021 14:00


Mathematics, 20.04.2021 14:00

Mathematics, 20.04.2021 14:00

Computers and Technology, 20.04.2021 14:00

Mathematics, 20.04.2021 14:00